Figures & data
Table 1. Effect of pretreatment on essential oil yield of leaves.
Figure 1. Antifungal activity against B. cinerea on various concentration of essential oils. B. cinerea cultured on PDB within 6 days and plates were incubated in controlled environment champers maintained at 25 °C. Values represent the mean ± SD (n = 3). Means followed by the same letters are not significantly different by Duncan’s test (p < 0.05).
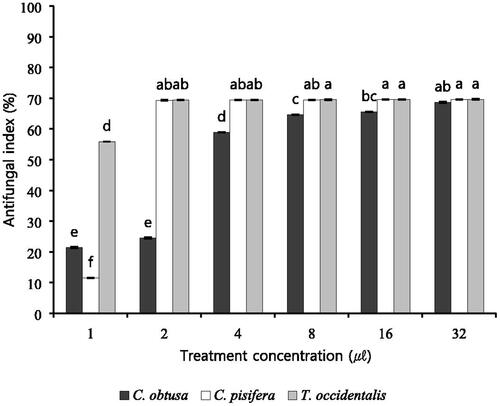
Figure 2. Impacts on various concentrations of three essential oils. (A) T. occidentalis. (B) C. obtuse. (C) C. pisifera. Colony growth (mm) of B. cinerea raised on PDB within 9 days. And plates were incubated in controlled environment champers maintained at 25 °C. Values represent the mean ± SD (n = 3). Means followed by the same letters are not significantly different by Duncan’s test (p < 0.05).
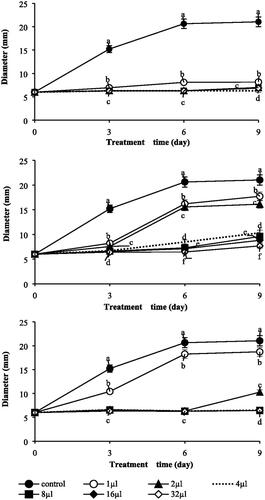
Figure 3. Capillary gas chromatogram of three species leaves volatiles obtained by steam distillation. (A) C. cbtusa, (B) C. pisifera, and (C) T. occidentalis. The GC column was a 60 mm × 0.25 mm × 0.25 μm i. d. HP-1 fused silica capillary column. The peak numbers correspond to the numbers in . 1: I-Phellandrene, 2: Tricyclene, 3: α-Pinene, 4: Camphene, 5: Sabinene, 6: β-Pinene, 7: β-myrcene, 8: Myrcene, 9: 3-Carene, 10: Benzene, 11: Bicyclo, 12: Thujone, 13: α-Thujone, 14: Camphor, 15: 3-Cyclohexen-1-1-ol, 16: Verbenol, 17: Benzenemethanol, 18: 3-Cyclohexen-1-methan, 19: 1,4-Methanoazulene, 20: Caryophyllene, 21: Bornyl acetate, 22: 2-Cyclohexen-1, 23: Terpinyl acetate, 24: trans-2-Caren, 25: α-Cedrane, 26: 1-methyl-4, 27: Widdrene, 28: methyl, 29: Naphthalene, 30: Cyclohexanemethanol, 31: Phenanthrene, 32: α-cedrol, 33: 2-Naphthalenemethanol, 34: 7-Methanoazulen, 35: β-Eudesmol, 36: Humulen, 37: Norkaur, 38: Limonene
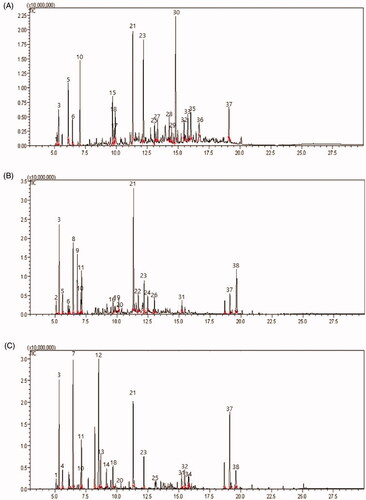
Table 2. Volatile compounds in steam-distilled essential oil of the leaves from C. obtuse and C. pisifera identified by gas chromatography-mass spectrometrya.
Figure 4. Putative bioactive compound profiled by GC-MS analysis. Terpinyl acetate (L); Bornyl acetate (R).
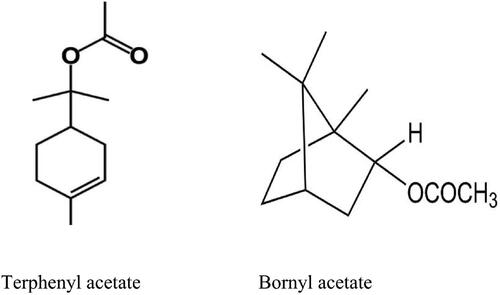
Table 3. Antifungal activity of terpinyl acetate, bornyl acetate, a mixture of two monoterpenes Antifungal activity according to each treatment concentration.
Figure 5. Antifungal activity of B. cinerea on various concentration of six individual monoterpene. B. cinerea raised on PDB within 6 days and plates were incubated in controlled environment champers maintained at 25 °C. Values represent the mean ± SD (n = 3). Means followed by the same letters are not significantly different by Duncan’s test (p < 0.05).
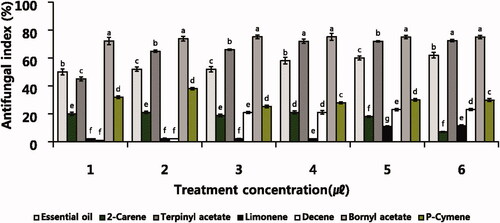
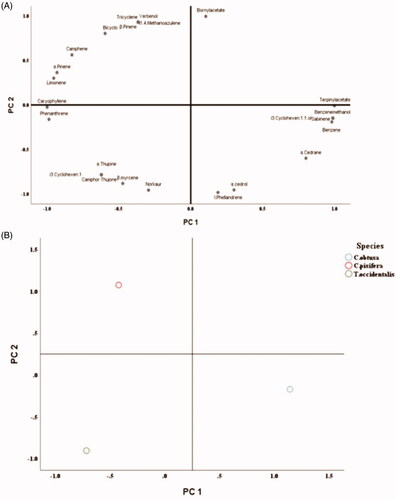
Figure 6. Antifungal activity of B. cinerea on various concentration of six individual monoterpene. A: Distribution divided into first principal component (PC1) and second principal component (PC2). B: Location of each species.