Figures & data
Figure 1. Schematic of ORF2 and tagged ORF2 fragments. The ORF2p molecule has multiple annotated domains important for retrotransposition. These include the enzymatically necessary endonuclease (EN: light blue) and reverse transcriptase (RT: purple) domains. Between the EN and RT domains is the Cryptic region (Cry: dark blue) and Z domain (Z: orange). Both contain amino acids essential to retrotransposition and ORF2p function. At the C-terminal end of the ORF2p is a cysteine-rich domain (Cys: yellow) that also contains amino acids essential to retrotransposition. EN-containing ORF2 fragments detailed here were generated in 2 formats: one with an N-terminal Gal4 tag and one with an N-terminal VP16 tag. VP16-tagged expression plasmids also contain a neomycin resistance gene. EN239Δ, EN269Δ, EN274Δ, EN289Δ, EN347Δ, and ENZ490Δ were also generated in an untagged format with a Hygromycin resistance gene in the expression plasmid. As described in materials and methods, reported domains are used as the body of the name, followed by the number corresponding to the terminal amino acid as it would be in the full length ORF2p, with the truncated ORF2p sequence denoted by a Δ.
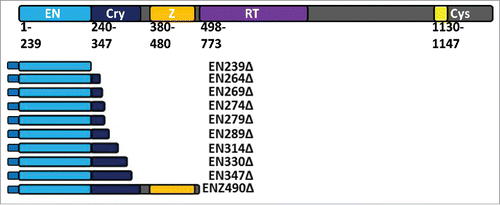
Figure 2. Chronic and Acute Toxicity of EN-containing ORF2 fragments. VP16-tagged and Gal4-tagged EN-containing ORF2 fragments are toxic to HeLa cells. (A) Chronic and Acute toxicity assays of EN-containing ORF2 fragments in HeLa cells. Amino acid that ORF2 fragment terminates in (ENΔ) denoted below graph bars for both functional (black) and nonfunctional (gray) EN-containing ORF2 fragments. Nonfunctional EN-containing fragments have catalytically necessary amino acids mutated in the EN domain (D205A, H230A). Error bars denote standard deviation (n = 3). Statistical significance was assessed using Student's t-test (*p < 0.05). (B) Grouping of chronic toxicity. Colony counts for EN264Δ and EN269Δ are not significantly different from one another. Colony counts for EN274Δ and EN279Δ are not significantly different from one another. Colony counts for EN264Δ and EN269Δ are significantly different from colony counts for EN274Δ and EN279Δ. Statistical significance was assessed using Student's t-test (*p < 0.05). (C) Grouping of acute toxicity. Colony counts for EN239Δ, EN264Δ and EN269Δ are not significantly different from one another. Colony counts for EN274Δ, EN279Δ, EN289Δ, EN314Δ, EN330Δ, EN347Δ, and ENZ490Δ are not significantly different from one another. Colony counts for EN239Δ, EN264Δ and EN269Δ significantly different from colony counts for EN274Δ, EN279Δ, EN289Δ, EN314Δ, EN330Δ, EN347Δ, and ENZ490Δ. Statistical significance was assessed using Student's t-test (*p < 0.05).
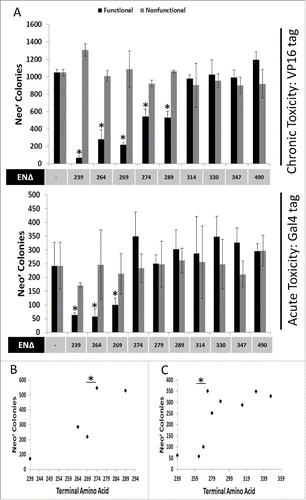
Figure 3. Subcellular distribution of Gal4-tagged EN-containing ORF2 fragments. Western blot analysis of EN-containing ORF2 fragments. Indicated Gal4-tagged EN-containing constructs were transiently transfected into HeLa cells and subjected to Western blot analysis. Both whole cell lysate and cytoplasmic and nuclear cell fractions were analyzed using commercially available anti-Gal4 antibodies, yielding a single band in each sample lane corresponding to the expected size of each ORF2p fragment. Control is cells transfected with empty vector. Cell fractions are indicated on the right. Molecular weight markers are indicated on the left. GAPDH (total cell lysate and cytoplasmic fraction) and Lamin A/C (L A/C: nuclear fraction) were used as loading controls.
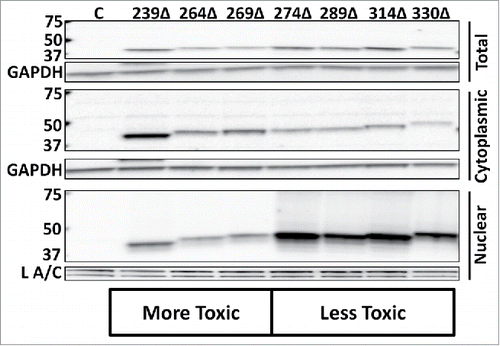
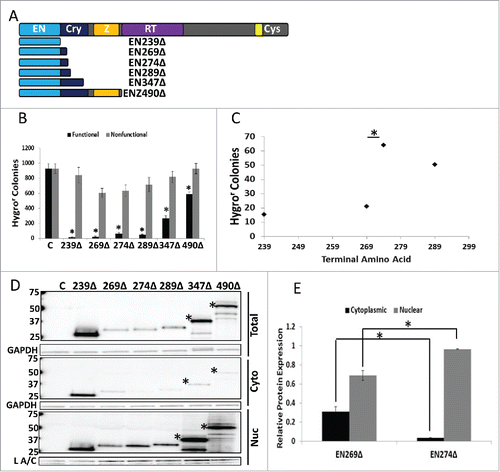
Figure 4. Analysis of Untagged EN-containing ORF2 Fragments: Selected untagged EN-containing ORF2 fragments behaved similarly to the corresponding VP16- and Gal4-tagged EN-containing ORF2 fragments in regards to cytotoxicity and subcellular distribution. (A) Schematic of untagged ORF2 fragments used in this experiment with ORF2 displayed above for reference. (B) Chronic toxicity of EN-containing ORF2 fragments. All ORF2 fragments were cytotoxic as compared to their nonfunctional controls. Error bars denote standard deviation (n = 3). Statistical significance assessed using Student's t-test (*p < 0.05). (C) Analysis of toxicity grouping of EN-containing ORF2 fragments. Analysis is identical to (*p < 0.05). (D) Western blot analysis of HeLa cells transiently transfected with indicated constructs using anti-ORF2p monoclonal antibodies. Both whole cell lysate and cytoplasmic and nuclear cell fractions were analyzed. Highest molecular weight band in each sample corresponds to expected size of each construct, with larger EN-containing fragments undergoing processing to yield smaller products (*: EN347Δ and ENZ490Δ). Control (C) is cells transfected with empty vector. Cellular fraction is indicated on the right. Molecular weights are indicated on the left. GAPDH (total and cytoplasmic (cyto)) and Lamin A/C (L A/C: nuclear (nuc)) used as loading control. Relative distribution in subcellular compartments of EN269Δ and EN274Δ is quantified in (E). (E) Quantitation of relative distribution of EN-containing ORF2 fragments. Signal intensity of EN-containing ORF2p fragments in each compartment was normalized to loading control. Normalized signal was summed for each cellular compartment and proportion protein expression quantitated per compartment (Example for Cytoplasmic: Normalized Cytoplasmic Signal/(Normalized Cytoplasmic Signal+Normalized Nuclear Signal)). Error bars denote standard deviation (n = 3). Statistical significance was assessed using Student's t-test (*p < 0.05).