Figures & data
Figure 1. (A) Cryo-EM micrograph showing T7 phages attached to LPS layers during the ejection reaction.Citation11 (B) Denaturing gel electrophoresis of T7 phages before (right) and after (left) incubation with rough LPS.Citation11
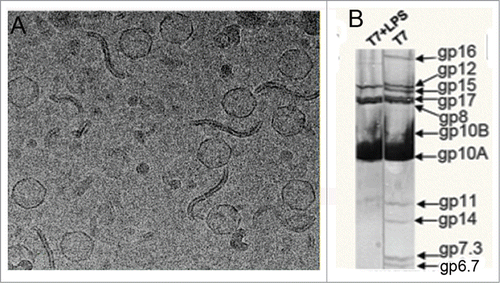
Figure 2. (A) Side view and (B) end-on view of the 3-dimensional reconstruction of the T7 tail before (left, EMD-5689Citation5) and after (right, EMD-2717Citation11) DNA ejection at ˜20 Å resolution. The capsid, connector and gatekeeper were colored in gray while components playing the main conformational changes during DNA ejection, the fibers and the nozzle were colored in blue and multiple colors, respectively.
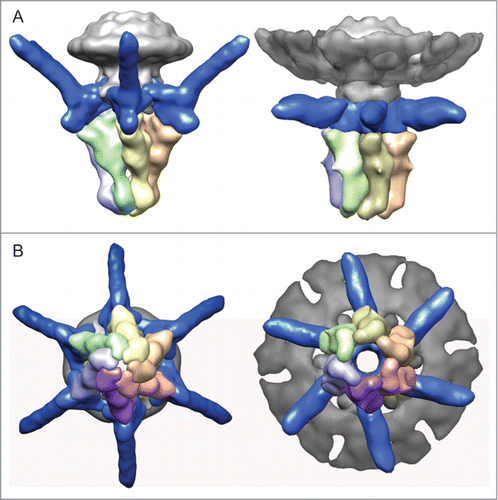
Figure 3. (A) analysis of T7 infection of E. coli WT and porine-defective mutant strain. The image shows lysis plaques obtained after plating T7 bacteriophage with E. coli WT (left) and mutant ΔlamB ΔompF::Tn5 ΔompA ΔompC (37) (right) at a moi of 3. (B) One-step growth of phage T7 on E. coli WT (solid line) and porine-defective E. coli mutant (dashed line). Titer was tested every 10 min; the inset shows the same experiment tested every 2.5 min. (C) Adsorption of viruses to the bacterial surface. Micrographs of 2% (w/v) PTA negatively-stained samples showing T7 particles incubated with E. coli WT bacteria (left) and porine-lacking mutant (right) at a moi of 100 for 7.5 min. The arrow indicates an emptied virus.
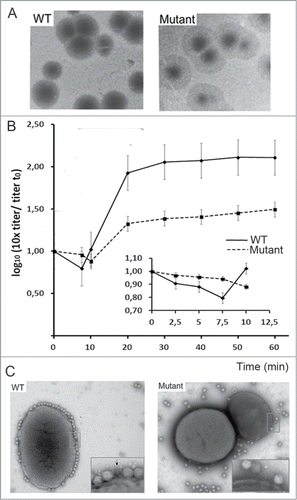
Figure 4. (A) Adsorption of phage T7 to immobilized cell wall proteins by the virus overlay protein binding assay (VOPBA). E. coli vesicles were obtained after osmotic shock and Frech preasure cell (Thermo Scientific) treatment to the bacteria, the pellet of the cellular debris was then purified in a 60% (w/v) sucrose cushion. Membrane proteins were solubilized from E. coli vesicles in 1.25% (w/v) N-dodecyl β-d-maltoside. VOPBA was performed as describedCitation23 with some modifications. Solubilized membrane proteins were loaded in a 5.5% native polyacrylamide gel and stained with Coomassie blue or electroblotted (left). Nitrocellulose membranes were incubated alone (control) or with 5 ml of 1011 p.f.u./ml T7 particles, then incubated with anti-gp10 capsid protein antibody, and developed with an enhanced chemilumiscence kit (ECL, GE Healthcare). The band for the reactive protein complex in native conditions was subjected to a second-dimension 17% SDS-PAGE in denaturing conditions and treated as above. (B) The 2 reactive bands were identified by mass spectrometry as OmpA and OmpF as described in . Protein lenght of OmpA (up) and OmpF (bottom) are represented with matched parts depicted in gray.
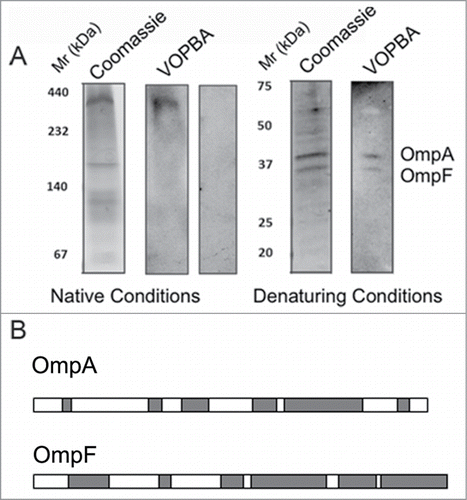
Table 1. Mass spectrometric analisys results of VOPBA detected gel bandsFootnotea
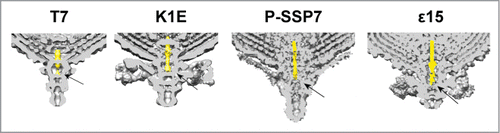
Figure 5. Cross section of the 3-dimensional volumes of T7 (EMD-5689Citation15), K1E (EMD-1333Citation32), P-SSP7 (EMD-1715Citation31) and ϵ15 (EMD-1175Citation33). The internal channel DNA fragment has been colored in yellow. The arrow points to the fiber-gatekeeper interface region.