Figures & data
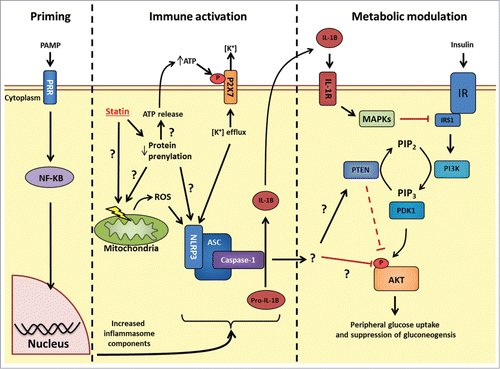
Figure 1. Possible mechanisms linking statin-induced NLRP3 inflammasome activation and insulin resistance. Priming: Following PRR stimulation, NF-κB stimulates transcriptional events that increase levels of the inflammasome (such as NLRP3) and inflammasome effectors (such as pro-IL-1β). Immune activation: HMGCR inhibition with statins causes pleotropic effects through decreased protein prenylation. Decrease in protein prenylation is a suspected cause for signals to promote increase NLRP3 inflammasome activity, but how these signals conspire to activate this inflammasome is not fully understood. Statins have been shown to cause mitochondrial membrane dysfunction, increase intracellular reactive oxygen species and also promote release of cellular ATP. Extracellular ATP can bind to the P2X7 receptor and promote potassium (K+) efflux, a key trigger for increased NLRP3 inflammasome activity. The identity of the prenylated protein(s) responsible for statin-induced inflammasome activation is not known. Following inflammasome activation, caspase-1 cleaves pro-IL-1β to biologically active IL-1β. Metabolic modulation: The connection between NLRP3 inflammasome activation and insulin signaling may occur through either IL-1β-mediated inflammation and activation MAPKs (JNK, ERK, p38) which inhibit insulin signaling at the level of receptor substrate-1 (IRS1); or through an unknown target of caspase-1 which may alter insulin signaling at the level of phosphatase and tensin homolog (PTEN) or another site such as AKT phosphorylation. PRR, pattern recognition receptor; NLRP, NOD-like receptor family, pyrin domain containing; HMGCR, HMG-CoA reductase; IL-1β, interleukin-1 β; P2 × 7, P2X purinoceptor 7; PI3K, Phosphoinositol-3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PDK1, phosphoinositide-dependent kinase-1; MAPK, Mitogen activated protein kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular regulated mitogen-activated protein kinase; IR, insulin receptor.