Figures & data
Figure 1. Isolation and characterization of human adipose tissue from various depots. (A) Schematic of procedure for isolation of adipose tissue and preadipocytes from adult human subcutaneous and omental as well as fetal interscapular sites. (B) RT-PCR of RNA isolated from adult human subcutaneous (sub), fetal interscapular (fiBAT) and adult human omental (ome) adipose tissue. UCP-1, uncoupling protein-1; ZIC1, zinc finger of the cerebellum1; PGC1-α, peroxisome proliferator-activated receptor gamma co-activator 1-α; PPAR-α, peroxisome proliferator-activated receptor α; LHX, LIM homeobox; PPAR-γ, peroxisome proliferator-activated receptor gamma.
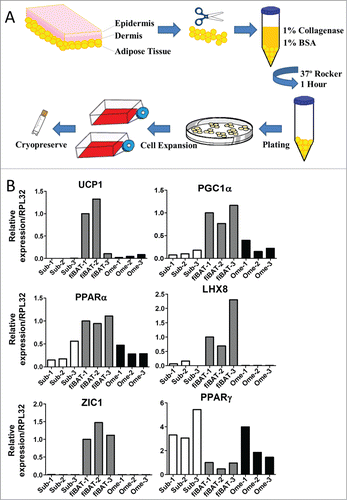
Figure 2. Morphologic appearance of cultured primary human adipocytes. Cryopreserved primary adult human subcutaneous and omental as well as primary fetal human interscapular pre-adipocytes were thawed and plated on tissue culture dishes. Cells were grown to confluence and then differentiated. (A) Phase-contrast images of adipocytes during various stages of in vitro differentiation. (B and C) Appearance of cells after staining for lipid using BODIPY (B) and oil-red-o (C).
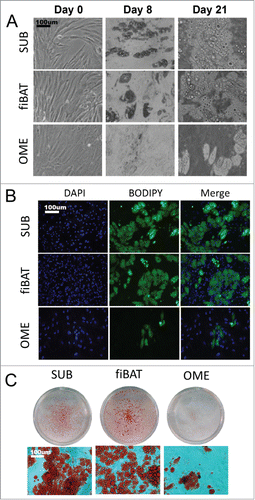
Figure 3. Molecular characterization of cultured primary human adipocytes. Adult human primary subcutaneous and omental pre-adipocytes and fetal human interscapular pre-adipocytes were differentiated in culture. Total RNA was extracted at various times during differentiation for RT-PCR analysis of common adipogenic markers (A) and BAT markers (B). The expression of these markers in fiBAT and interscapular adipose tissue is compared in (C). Total protein was isolated from adipocytes at Day 0 and Day 21 of differentiation for Western blot analysis of UCP-1 (D), PGC1α and FABP4 expression (E). * (P < 0.05) Gene expression level in BAT in comparison with SUB, analyzed by Student's t-test. CIDEA, cell death-inducing DFFA-like effector a.
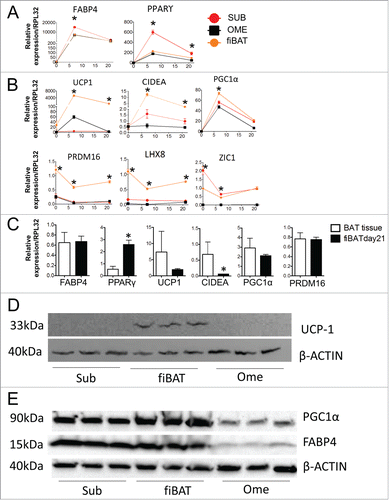
Figure 4. Metabolic characterization of cultured primary human adipocytes. Cryopreserved adult human primary subcutaneous and omental as well as primary fetal human interscapular pre-adipocytes were thawed and plated on tissue culture dishes. Cells were grown to confluence and then differentiated. (A) At day 21 of differentiation cells were stained with mitotracker. The redness in each picture is quantified with ImageJ and the quantification results are displayed in the right panel. n = 3. (B) Day 21 fiBAT cells were treated with 1 μM norepinephrine (NE) for 3 hours and real-time PCR was performed to examine the expression of UCP1 and PGC1α. (C) Day 21 primary subcutaneous and interscapular adipocytes were incubated with [1−14C] oleate. Label incorporation in CO2 was measured to assess rates of fatty acid oxidation. (D and E) The metabolic profile of cultured adipocytes (day 21) was assessed using a Seahorse XF24 extracellular flux analyzer. (D) Representative curves of the oxygen consumption rates (OCR) of adipocytes in their basal states and when treated with drugs to dissect the multiple components of the respiration process. (E) Quantitation of the OCRs from part C. * Significant (P < 0.05) ORC level for fiBAT in comparison with SUB, analyzed by Student's t-test.
![Figure 4. Metabolic characterization of cultured primary human adipocytes. Cryopreserved adult human primary subcutaneous and omental as well as primary fetal human interscapular pre-adipocytes were thawed and plated on tissue culture dishes. Cells were grown to confluence and then differentiated. (A) At day 21 of differentiation cells were stained with mitotracker. The redness in each picture is quantified with ImageJ and the quantification results are displayed in the right panel. n = 3. (B) Day 21 fiBAT cells were treated with 1 μM norepinephrine (NE) for 3 hours and real-time PCR was performed to examine the expression of UCP1 and PGC1α. (C) Day 21 primary subcutaneous and interscapular adipocytes were incubated with [1−14C] oleate. Label incorporation in CO2 was measured to assess rates of fatty acid oxidation. (D and E) The metabolic profile of cultured adipocytes (day 21) was assessed using a Seahorse XF24 extracellular flux analyzer. (D) Representative curves of the oxygen consumption rates (OCR) of adipocytes in their basal states and when treated with drugs to dissect the multiple components of the respiration process. (E) Quantitation of the OCRs from part C. * Significant (P < 0.05) ORC level for fiBAT in comparison with SUB, analyzed by Student's t-test.](/cms/asset/4768ed6a-8ac8-45d5-b121-741769a6f35f/kadi_a_1042192_f0004_c.gif)
