Figures & data
Figure 1. The IL-22 / IL-22R axis in obesity and cancer. Analysis of tissues from lean and obese mice reveals that in WAT IL-22 expression by infiltrating leukocytes is increased in obesity (arrows) and that in tumors IL-22 signal is associated with tumor cells and that IL-22R expression by tumor cells is increased in obesity (arrowheads). Mesenchymal and endothelial cells are GFP-positive; leukocytes and malignant cells are GFP-negative. The experiment was performed in C57BL/6-Tg(UBC-GFP)30Scha/J mice that had received a bone marrow transplant from B6.Cg-Tg(ACTB-mRFP1)1F1Hadj/J mice as described.Citation8,44 Mice had been fed with chow (lean) or 58 kcal% (fat) diet to induce DIO for 4 months (obese). EO771 cells were then grafted into the mammary fat pad and tumors were allowed to form. Formalin-fixed paraffin-embedded tissues were sectioned and analyzed by immunofluorescence as describedCitation49,50 using anti-IL-22 bs-2623R (Bioss, 1:100) and anti-IL-22R bs-2624R (Bioss, 1:200) antibodies, followed by secondary Alexa 488-conjugated and Cy3-conjugated antibodies. Nuclei were visualized with DAPI staining. Scale bar, 50 µm.
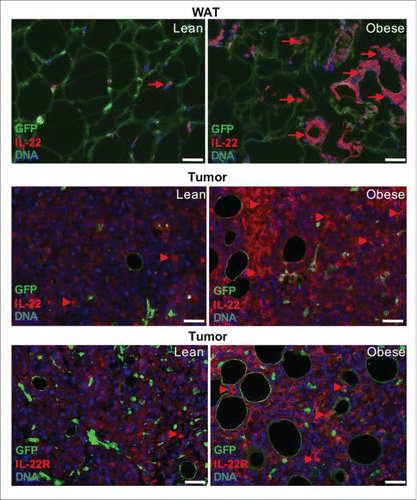
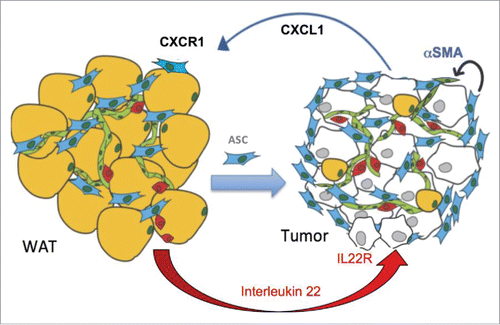
Figure 2. A working model for the cytokine cascade recruiting ASC to tumors. In obesity, adipocyte (yellow) death recruits leukocytes (red) that secrete IL-22. This cytokine activates its receptor IL-22R in malignant epithelial cells, downstream of which the chemokine CXCL1 is expressed. ASC (blue) express CXCL1 receptor, CXCR1. Obesity-induced secretion of CXCL1 by cancer cells creates a chemokine gradient that enables ASC trafficking to tumors via CXCR1. Upon tumor recruitment, ASC promote cancer in part via stimulating the endothelium (green) by αSMA expressed downstream of CXCR1 signaling.