Figures & data
Table 1. Comparison of each SGLT2 inhibitor in the potency on SGLT2 and selectivity over SGLT1 in the kidney.
Figure 1. Obesity-related macrophage polarization and insulin resistance. In a lean state, M2 macrophages are the primary resident macrophages and maintain insulin sensitivity. In contrast, excess calories or a sedentary lifestyle cause adipocyte hypertrophy, which initiates secretion of CCL2 and CCL5, leading to the recruitment of circulating monocytes in adipose tissues. Subsequently, CCR2+ macrophages accumulate and presumably maintain inflammation as M1 macrophages in obese adipose tissue. Once these ATMs are present and active, they maintain a vicious cycle involving ATM recruitment and the production of inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, in conjunction with adipocytes and other infiltrated immune cells. These secreted proinflammatory cytokines subsequently cause inflammation and insulin resistance in adipose tissue, liver, and skeletal muscle.
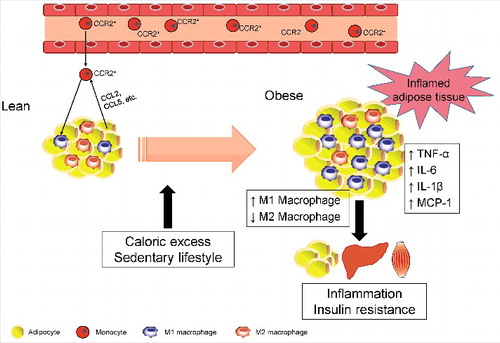
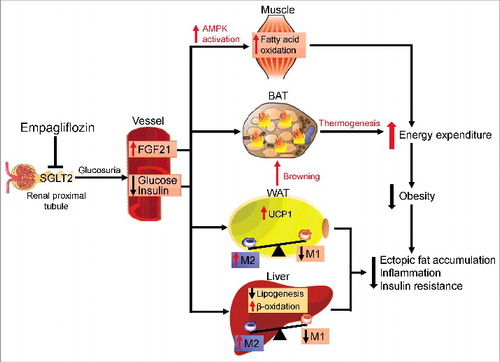
Figure 2. Protective effects of empagliflozin in high-fat diet-induced obese mice. Inhibiting SGLT2 with empagliflozin directly decreases blood glucose levels, leading to the following: (1) Empagliflozin promotes fat utilization by enhancing AMPKα and ACC phosphorylation in skeletal muscle and increasing hepatic and plasma levels of FGF21. (2) Empagliflozin enhances browning and thermogenesis in WAT and BAT, which results in increased energy expenditure. (3) Empagliflozin improves insulin sensitivity by polarizing M2 macrophages in fat and liver.