Figures & data
Figure 1. Analysis of iNKT cells and Treg cells after 1 week of HFD feeding. A: The mRNA level of IL-2 in sorted iNKT cells (TCRβ+PBS57/CD1d tetramer+) from EATs of HFD-fed CDldf/f and CDldADKO mice. EATs were pooled from 15 mice per group. B: The percentage of Treg cells (TCRβ+CD4+Foxp3+) among CD4 T cells in EATs. n = 5. C: The percentage of Treg cells in EATs during NCD and HFD feeding period. N = 4–5. *p < 0.05 and **p < 0.0l. 8-week-old mice were fed NCD or 60% HFD for the indicated time periods.
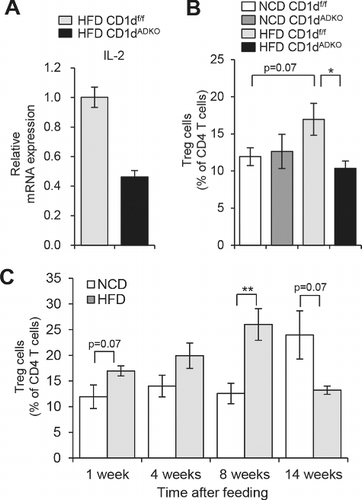
Figure 2. Proposed model for the interaction between adipocyte CDld and adipose iNKT cells in the regulation of adipose tissue inflammation with progressive obesity. In lean adipose tissue, adipocytes highly express CDld molecules which play a crucial role in the maintenance of adipose iNKT cell population. Upon HFD feeding, adipocytes present obesity-related lipid antigens via CDld molecules, which leads to iNKT cell activation and stimulates anti-inflammatory cytokine secretion from adipose iNKT cells. Concurrently, activation-induced cell death (AICD) is occurred in part of activated iNKT cells, which results in reduced number of iNKT cells in adipose tissues. On the other hand, the significantly reduced CDld expression on adipocytes from severely obese adipose tissues weakens adipose iNKT cell stimulation. Elevated inflammatory responses which are associated with the impairment of anti-inflammatory responses accelerate adipose tissue dysfunction including FFA release. Increased circulating FFAs could accumulate in the liver.