Figures & data
Figure 1. The role of omental adipocytes in tumour development and metastasis.
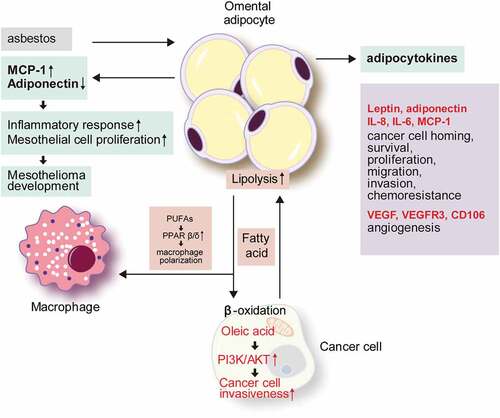
Figure 2. The role of bone marrow adipocytes in solid tumour metastasis and hematologic tumour development.
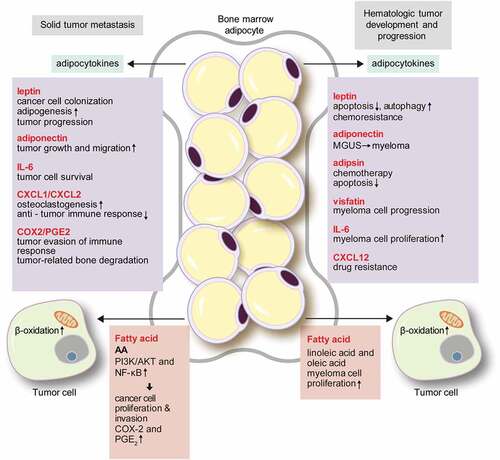
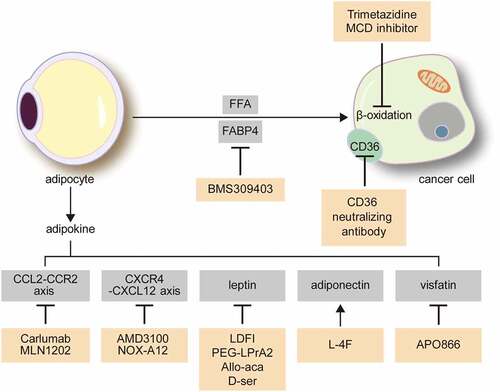
Figure 3. Possible treatment targets for the interaction between cancer cells and adipocytes in the omentum and bone marrow.