Figures & data
Figure 1. Schematic diagram of protein sample preparation from fat tissues for Western blotting. Crude proteins from lipid-rich tissues, such as epididymal or inguinal white adipose tissues (eWAT or iWAT, respectively), were prepared by chemical (lysis buffer) and physical (homogenization by shaking beads) lysis followed by centrifugation. After centrifugation, the lipids (fat cake) are overlaid on top of the protein-containing supernatant. To eliminate lipids from the protein fraction, an acetone precipitation method was adopted, as indicated in the blue box (see the details in the methods section).
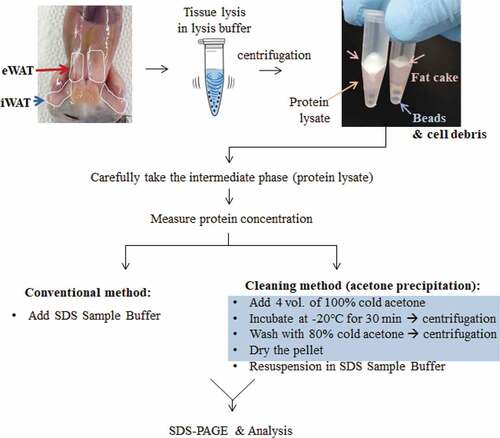
Figure 2. Resolution comparison for iWAT proteins. iWAT from mice exposed to cold conditions (Cold, n= 3) or housed at room temperature (RT, n= 3) were lysed in RIPA buffer (a) or adipocyte lysis buffer (b) and prepared by the conventional method (30 μg/well) or the cleaning method (60 μg of starting weight/well). Blots were probed with antibodies against UCP1 or two different loading controls, Actin and HSP90. Protein lysates from interscapular brown adipose tissue (iBAT) from UCP1 knock-out (UCP1 -/-) or control mice (UCP1 +/+) were prepared by a conventional method and used as a negative or positive control for UCP1, respectively. Compared to iWAT, only 10% of the iBAT lysate was loaded (3 μg/well).
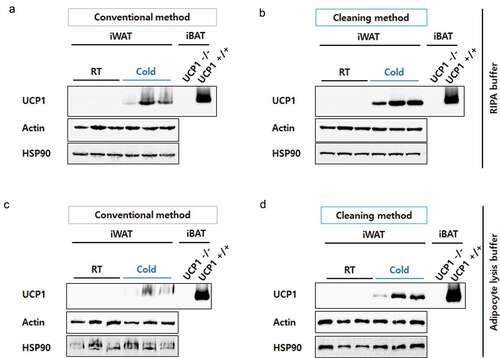
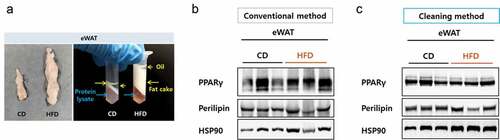
Figure 3. Resolution comparison for eWAT proteins. (a) Left, size comparison of one side of eWAT from mice fed a chow diet or a high-fat diet. Right, separated layers after a centrifugation during a protein preparation. Blue arrows indicate protein lysate component and yellow arrows indicate fatty components. (b) eWAT lysed in RIPA buffer and protein lysates were prepared by either the conventional (b) or the cleaning method (c). Blots were probed with antibodies against PPAR gamma or perilipin and HSP90 as a loading control.