Figures & data
Figure 1. In human breast tumors, TEM can be identified solely based on CD14 immunostaining. (A) Immunofluorescence staining and confocal analysis of human BC tumor sections showing CD14, TIE-2 and VEGFR-1 triple positive cells. Representative image from 16 patients; (B) CD14 and TIE-2 expression correlation analysis. The dot plot (left panel) represents all tiles for one representative image; the box plot (right panel) shows the Spearman’s rank correlation coefficient (ρ) for CD14 and TIE-2 expression in 11 patients. In the dot plot, 54% of CD14+ tiles and 70% of TIE-2+ tiles were above the threshold. 100% of CD14+ tiles were TIE-2+ which means that the high correlation (ρ = 0.844) applied to all CD14+ tiles; (C) CD14 and VEGFR-1 expression correlation analysis for 11 patients. In the dots plot, 55% of CD14+ tiles and 75% of VEGFR-1+ tiles were above the threshold. 100% of CD14+ tiles are VEGFR-1+ which means that the high correlation (ρ = 0.852) applied to all CD14+ tiles; (D) Example of TIE-2 expression in TEM, clusters of BEC (arrow heads) and blood vessels (right panel) shown in confocal microscopy images of BC sections. TEM and blood endothelial structures were stained with CD14 and CD31, respectively; (E) Example of immunofluorescence labeling of CD14, VEGFR-1 and TIE-2 in sections containing non-neoplastic breast tissue adjacent to tumor tissues. CD14+ cells (arrows) are VEGFR- and TIE-2-. Non-neoplastic (panel E) and tumor tissue (panel A) were stained and imaged simultaneously under the same conditions and thus intensities of the expression of TIE-2 and VEGFR-1 signals can be compared. Representative images from four patients. Scale bars: 25 μm.
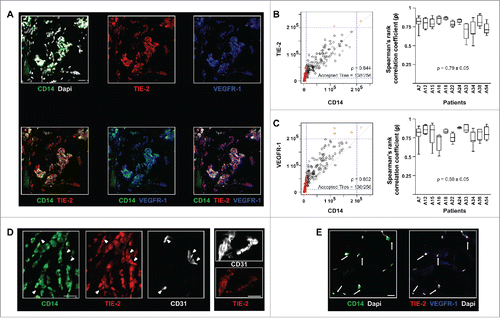
Table 1. Clinical and pathological features of tumors and patients (n = 31).
Figure 2. TEM are highly angiogenic cells supporting breast tumor growth. Quantification of the volume and of the vascularization of PDX tumors two weeks post-injection in the tumor vicinity of autologous tumor TEM (Tumor TEM mice) or buffer and magnetic beads (No cell, control mice). Pairs of mice showing comparable tumor volume have been used. Scale bar: 50 μm.
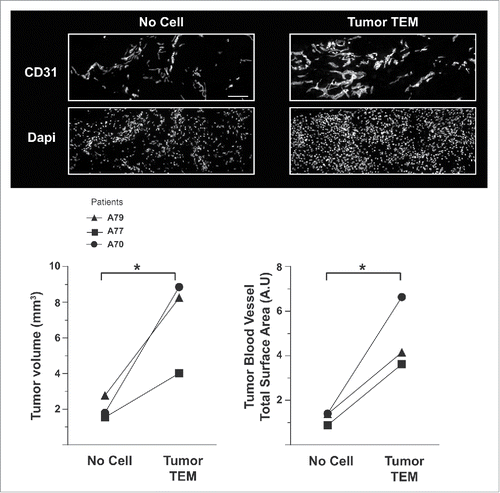
Figure 3. TEM carry a lymphatic phenotype in human breast tumor. The expression of (A) the lymphatic markers VEGFR-2, LYVE-1, VEGFR-3, Podoplanin, and PROX-1 and (B) the VEGFR co-receptors Neuropilin-1 and Neuropilin-2 was examined in breast tumor sections by confocal microscopy. Representative images from minimum five patients; (C) Some TEM formed small cell aggregates (10–100 cells); (D) PROX1 expression analysis was performed by RT-PCR from mRNA isolated from mouse cells (negative control), LEC in culture (positive control) and from TEM (CD14+CD45+ cells) sorted by flow cytometry from dissociated breast tumor. One representative gene expression profile from 10-cell samples from one patient is shown out of 32 cell samples from four patients (8 samples/patient of 10 cells each); (E) Immunofluorescence labeling in sections of non-neoplastic breast tissue adjacent to tumor tissues shows no detectable expression of Podoplanin and VEGFR-3. Non-neoplastic (panel E) and tumor tissue (panel A and C) were stained and imaged simultaneously under the same conditions and thus intensities of the expression of Podoplanin and VEGFR-3 signals can be compared. Scale bars (A-C and E): 25 μm, (A) higher magnification, scale bar: 10 μm.
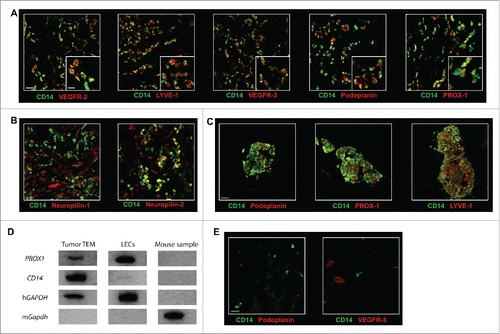
Figure 4. In BC tumors, TEM are associated with patchy and poorly structured blood vessels. (A) TEM spatial relationship to small and large vessels. Scale bar: 100 μm, representative image from 16 patients; (B) TEM were found located in the proximity of small blood vessels, rather than large vessels (t test, *** p ≤ 0.0001); (C) In BC tumors, the vast majority of large vessels (arrow heads) stain positive for α-SMA while small vessels do not. Scale bar: 50 μm.
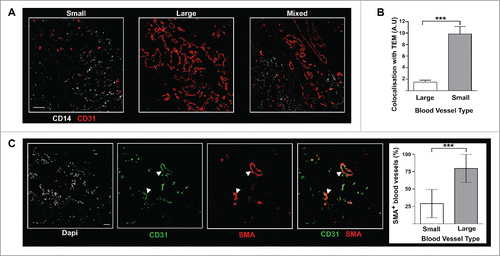
Figure 5. In BC tumors, TEM are associated with lymphatic structures. (A) TEM (arrow, CD14+LYVE-1+PROX-1+ cells) were associated with lymphatic vessels (arrowhead, CD14-LYVE-1+PROX-1+ cells); (B) Immunofluorescence labeling of TEM-containing lymphatics (arrows) with VE-cadherin; (C) Immunofluorescence labeling in sections of non-neoplastic breast tissue adjacent to tumor tissues shows no TEM association with LYVE-1+ lymphatic vessels. Scale bars: 25 μm. Representative image from seven patients.
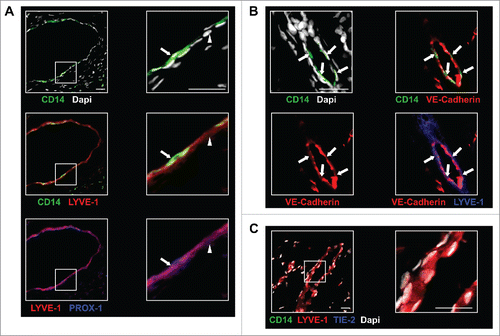
Figure 6. TEM lymphangiogenic activity is supported by cross-talk between VEGFR and TIE-2 pathways. (A) In vivo corneal vascularization assay was used to assess the lymphangiogenic activity of TEM isolated from human breast tumors. Lymphatic vessels emerging from the peripheral limbal vasculature were labeled with LYVE-1-specific antibodies in sagittal sections of mouse eyes. Double-headed and single arrows depict cornea and iris respectively. Scale bar: 100 μm; (B) Confocal microscopy images of TEM sorted from dissociated breast tumor showing expression of VEGF-A, -C and -D. Scale bar: 5μm; (C) TEM isolated from dissociated breast tumors were exposed to TIE-2- and VEGFR-specific kinase inhibitors and their hem- and lymphangiogenic activities were measured in vitro using a sprouting assay of HUVEC and LEC respectively (t test, *** p <0.001, **** p < 0.0001).
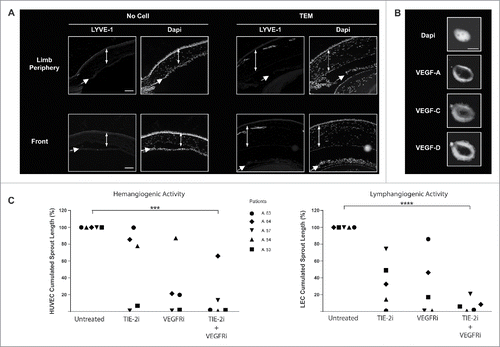
Table 2. Pathological features of the tumors used for each experiment.
