Figures & data
Figure 1. IL-10 induction after vaccination with tumor antigens + Imiquimod in tumor-bearing mice. C57BL/6 mice (n = 4–6/group) with 5 mm B16-OVA tumors were vaccinated by i.t. injection of OVA plus topical application of Imiquimod. (A) Two days later tumors were obtained, homogenized and IL-10 measured by ELISA. (B) Serum was also obtained at the day of sacrifice and IL-10 was measured, including also samples from control non-tumor bearing mice (naïve). (C) Mice bearing 5 mm TC-1 P3(A15) tumors were immunized with EDA-HPVE7 plus Imiquimod and serum IL-10 measured as in B. Results are representative of two-three independent experiments (*, P <0.05; **, P < 0.01).
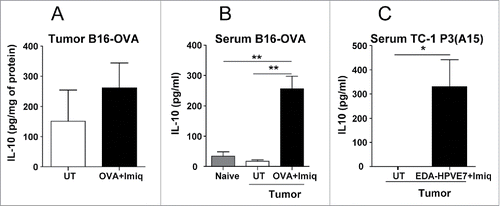
Figure 2. IL-10 blockade enhances the antitumor effect of therapeutic vaccination in B16-OVA and TC-1 P3(A15) tumor-bearing mice. C57BL/6 mice (n = 8/group) bearing 5 mm B16-OVA (A) or TC-1 P3(A15) (B) tumors were vaccinated with three weekly cycles of topical Imiquimod administration plus i.t. immunogen injection (OVA or EDA-HPVE7, respectively), combined with i.p. injection of neutralizing anti-IL-10R antibodies or an isotype control. Control groups of animals treated with anti-IL-10R or isotype antibodies were also included. Tumor growth and animal survival was monitored twice per week. Results are representative of two independent experiments.
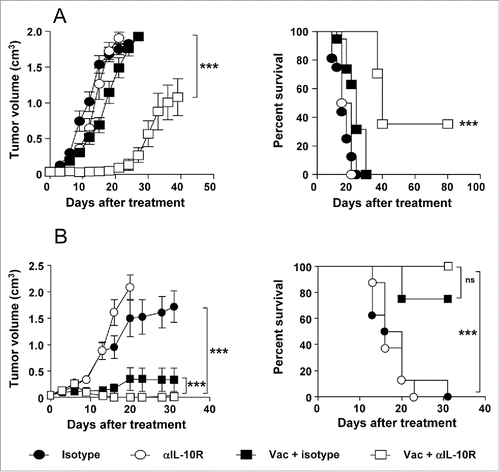
Figure 3. IL-10 blockade enhances vaccine-induced antitumor innate and adaptive responses in tumor-bearing mice. C57BL/6 mice (n = 4–6/group) bearing 5 mm B16-OVA tumors received a single vaccination with topical Imiquimod administration plus i.t. OVA injection, combined with i.p. injection of neutralizing anti-IL-10R antibodies or an isotype control. (A) They were sacrificed 2 d after vaccination and CD86 and intracellular IL-12 were determined by flow cytometry in DC from tumor-draining lymph nodes. (B) In the same animals as in A, lymphocytes were cultured with the NK-sensitive YAC-1 cells line and IFN-γ production measured by ELISPOT. (C) Equivalent groups were sacrificed one week after vaccination and cells from tumor-draining lymph nodes were stimulated with OVA(257–264) peptide or B16-OVA tumor cells to measure IFN-γ-producing cells by ELISPOT. (D) OVA(257–264)-specific CD8+ T cells from mice shown in C were analyzed by flow cytometry measuring the percentage of IFN-γ+TNF-α+ and IFNγ+TNF- α +IL-2+ CD8+ T cells after 5 h of stimulation with OVA(257–264). (E) Mice with B16-OVA tumors (n=10/group) were depleted of CD8+, CD4+ or NK cells and vaccinated with OVA + Imiquimod plus IL-10 blockade. As controls mice were left untreated or vaccinated in the absence of any cell depletion. Tumor volume was measured in these animals. Results are representative of two independent experiments.
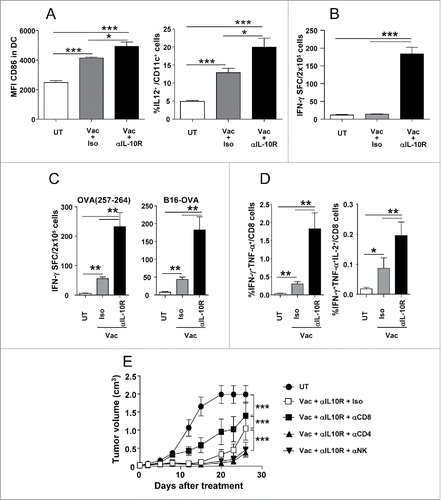
Figure 4. IL-10 blockade enhances (T)cell responses in an adjuvant-dependent manner. (A) C57BL/6 mice (n = 4 /group) immunized with OVA s.c. and topical Imiquimod cream application at the immunization site, combined with i.p. injection of isotype or anti-IL-10R antibodies. One week later their splenocytes were stimulated with CD8 epitope OVA(257–264) or OVA protein and IFNγ-producing cells evaluated by ELISPOT. (B) Mice were immunized with Imiquimod plus EDA-HPVE7 protein as in A with or without IL-10 blockade and responses against HPV E7(49–57) peptide were measured by ELISPOT. (C) C57BL/6 mice (n = 4) were immunized with OVA s.c. plus adjuvants CpG, poly(I:C) or anti-CD40 agonistic antibodies or with EDA-OVA plus control or anti-IL-10R blocking antibodies. One week later mice were sacrificed and their splenocytes were stimulated with CD8+ epitope OVA(257–264) and IFNγ-producing cells evaluated by ELISPOT. Results show the difference between peptide-stimulated minus unstimulated cells and are representative of two-three independent experiments.
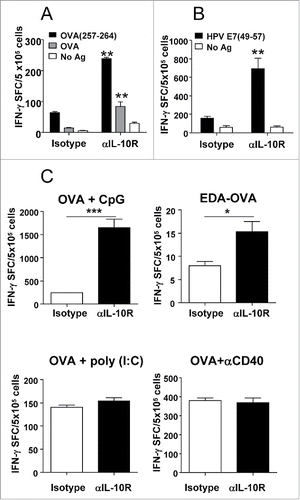
Figure 5. IL-10 blockade lacks antitumor effect when used in combination with adjuvants not inducing IL-10. C57BL/6 mice (n = 8/group) bearing 5 mm B16-OVA tumors were left untreated or received three weekly cycles of therapeutic vaccination consisting of i.t. injection of OVA plus poly(I:C) (A) or agonistic anti-CD40 antibodies (B), combined with i.p. injection of neutralizing anti-IL-10R antibodies or an isotype control. Tumor growth and animal survival was monitored twice per week. (C) Mice (n = 5/group) with 5 mm B16-OVA tumors were vaccinated with OVA+Imiquimod, OVA+poly(I:C) or left untreated. Two days later IL-10 content in tumor homogenates and serum was determined by ELISA. (D) Purified splenic CD11c+ DC from naïve C57BL/6 mice were stimulated in 96-well plates for 1 d with Imiquimod, CpG, poly(I:C), anti-CD40 or left unstimulated and IL-10 content in the supernatants was determined by ELISA. Results are representative of two-three independent experiments.
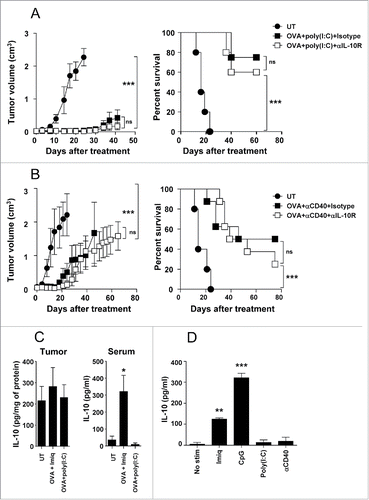
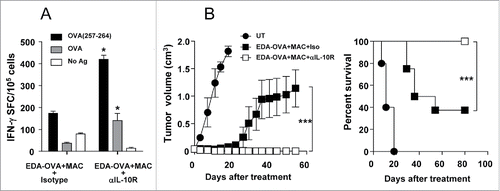
Figure 6. Inhibition of IL-10 when using a MAC-based therapeutic vaccine enhances antitumor responses resulting in complete tumor rejection. (A) C57BL/6 mice (n = 4) were immunized with EDA-OVA + MAC plus control or anti-IL-10R blocking antibodies. One week later mice were sacrificed and their splenocytes were stimulated with CD8 epitope OVA(257–264) or OVA protein and IFN-γ-producing cells evaluated by ELISPOT. (B) C57BL/6 mice (n = 8/group) bearing 5 mm B16-OVA tumors received three weekly cycles of vaccination with EDA-OVA + MAC, combined with i.p. injection of neutralizing anti-IL-10R antibodies or an isotype control. Tumor growth and animal survival was monitored twice per week. Results are representative of two independent experiments.