Figures & data
Figure 1. Mesothelioma patients have decreased numbers of blood DC subsets. Whole blood was stained and analyzed by flow cytometry. Representative dot plot (a) showing gating of leukocytes by size (FSC) and granularity (SSC). CD14+ monocytes, granulocytes and CD19+ B cells were further excluded by gating (b). Blood DC subsets were identified by high expression of BDCA-1 (c: mDC1), BDCA-3 (d: mDC2 and BDCA2 (c: pDC). Circulating mDC1 (e), and mDC2 (f) and pDCs (g) are shown as the number of DCs per mL of blood. Each dot represents an individual volunteer (mesothelioma: n = 48, age-matched controls: n = 36). ***p < 0.0001.
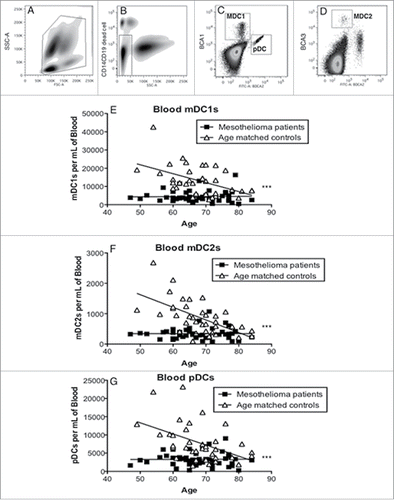
Figure 2. Mesothelioma-derived monocytes differentiate into iMoDCs. Blood monocytes from mesothelioma patients and age-matched volunteers were differentiated into iMoDCs using GM-CSF and IL-4 (a) and stained for analysis by flow cytometry. Representative plot (b) showing gating of large CD14- cells which were analyzed for expression of CD11c (c), CD40 (f), CD86 (i) and CD83 (l) with positive stained cells (gray filled) and unstained cells (unfilled). The percentages of iMoDCs positive for CD11c (d), CD40 (g), CD86 (j) and CD83 (m) and surface expression levels shown as MFIs of CD11c (e), CD40 (h), CD86 (k) and CD83 (n) in individual mesothelioma patients (n = 46) and age-matched controls (n = 27) is shown. *p < 0.05.
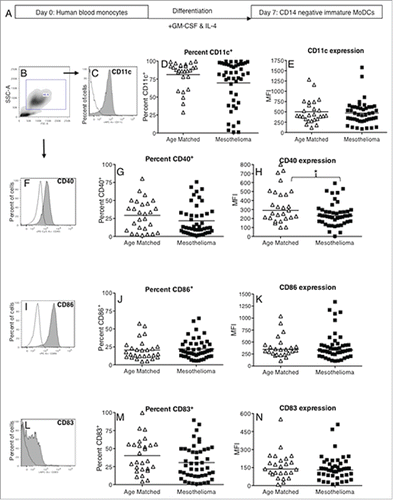
Figure 3. iMoDCs from mesothelioma patients have a reduced capacity to process antigen. Immature MoDCs from mesothelioma patients and age-matched controls were incubated for 1 h with DQ-Ovalbumin. Representative dot plot (a) showing gating of MoDCs based on size and granularity. The capacity to process antigen was determined by emission of a signal in the FITC channel (b) and measured by flow cytometry; gray histogram represents cells incubated with DQ-OVA, white histogram represents control cells that did not receive DQ-OVA. Pooled data (c) of the mean fluorescent intensity (MFI) indicating relative antigen-processing capacity of mesothelioma (n = 42) vs. age matched (n = 29) MoDCs is shown as mean ± SE. **p < 0.005.
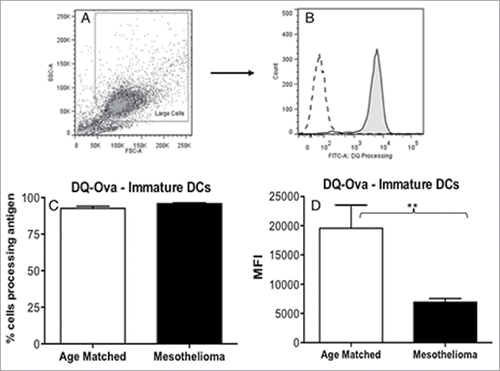
Figure 4. MoDCs from mesothelioma patients do not fully upregulate CD83, CD40 and CD86 in response to maturation stimuli. Immature MoDCs generated from mesothelioma patients and age-matched volunteers were stimulated with LPS (a) and cell surface molecules analyzed by flow cytometry. Pooled data of the percentages of cells positive for CD11c (b), CD40 (d), CD86 (f) and CD83 (h). Surface expression levels were measured and shown as MFIs of CD11c (c), CD40 (e), CD86 (g) and CD83 (i) in mesothelioma patients (n = 46) vs. age matched (n = 27) iMoDCs. Pooled data is shown as mean ± SE.
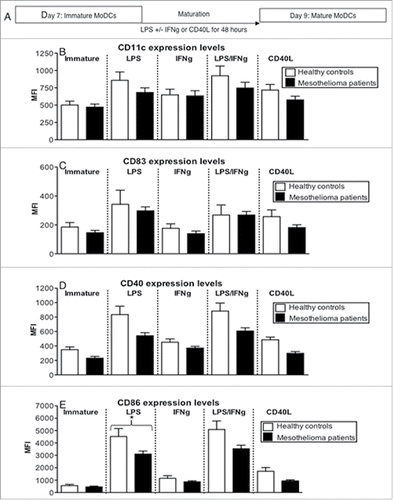
Figure 5. LPS-matured MoDCs from mesothelioma patients lose their capacity to process antigen. Immature and LPS+/-IFNγ or CD40L activated MoDCs from mesothelioma patients and healthy age-matched volunteers were incubated for 1 h with FITC-DQ-OVA. Pooled data of the percentage of DCs still processing antigen and MFIs indicating relative antigen-processing capacity is shown as mean ± SE from age-matched volunteers (n = 29) and mesothelioma patients (n = 42). *p < 0.05.
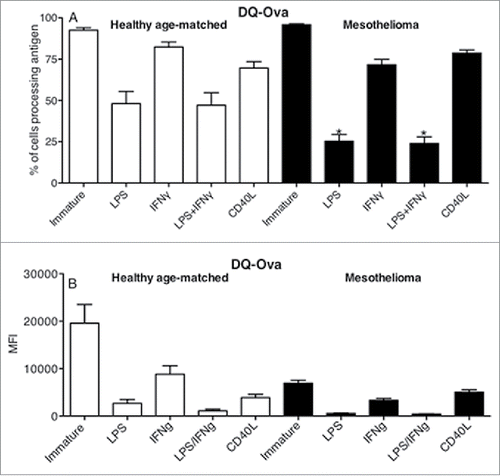
Figure 6. LPS-matured DCs mesothelioma induce T cell proliferation. Immature and LPS+/-IFNγ or CD40L-activated MoDCs were co-cultured with allogeneic CFSE-labeled lymphocytes for 7 d. Cells were collected, stained for CD4+ and CD8+ expression and analyzed by flow cytometry. Lymphocytes were gated by size and CD4+ or CD8+ expression and the percentage of proliferating cells of total cells determined. Pooled data from healthy age-matched (n = 19) vs. mesothelioma patients (n = 23) MoDCs is shown as mean ± SE.
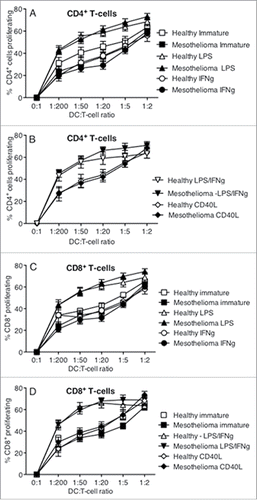
Figure 7. MoDCs from mesothelioma patients secrete the same or higher levels of cytokines in response to stimulation. Culture media from LPS+/-IFNγ or CD40L-stimulated MoDCs from mesothelioma patients and healthy age-matched controls were analyzed by CBA for cytokine production. Pooled data for IL-10 (a), VEGF (b), TNF (c), IL12p70 (d) and IFNγ (e) secreted by MoDCs from mesothelioma patients (n = 45) and age-matched controls (n = 14) is shown as mean ± SE.
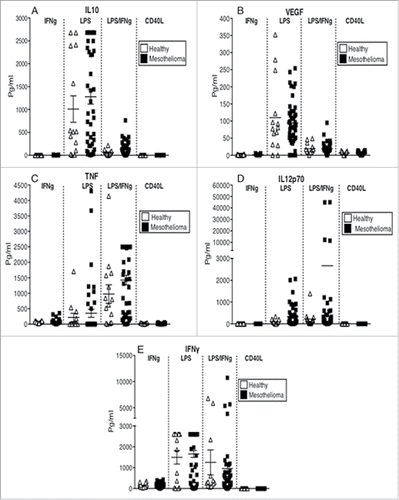
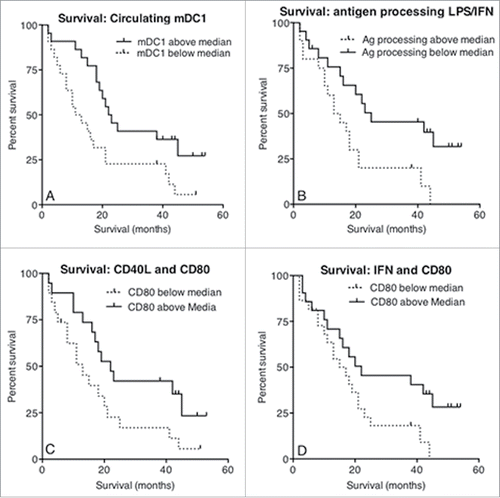
Figure 8. Increased survival correlates with circulating mDC1s and MoDCs that respond appropriately to maturational stimuli. Whole blood from mesothelioma patients was analyzed for blood DC subpopulations and the number of circulating MDC1s plotted against survival from time of blood collection (a). The percentage of mesothelioma patient-derived LPS/IFNγ stimulated MoDCs able to process antigen measured using the DQ-OVA assay was plotted against survival (b). The percentage of cells expressing CD80 following stimulation with CD40L was plotted against survival (c). Similarly, CD80 expression levels (MFI) following stimulation with IFNγ were plotted against survival (d). All p values were determined using the Log-Rank Test.