Figures & data
Figure 1. Viral replication and CXCL11 expression from the virus both in vitro and in vivo. AB12-luc cells were infected with vvDD-CXCL11 or vvDD. The infected cells were harvested 24 h post infection for RNA purification. The production of virus progeny from infected cancer cells at 48 h post infection was determined by plaque assay (A). Purified total RNAs were subject to RT-qPCR for quantitative detection of A34R mRNA (viral gene) (B) or CXCL11 (C). The quantity of secreted CXCL11 was measured by ELISA assay (D). For in vivo analyses (E), 4 × 105 AB12-luc cells were inoculated i.p. into naive BalB/c mice at day 0 and then injected i.p. with vvDD-CXCL11 or control virus vvDD (1 × 108 pfu/mouse) or PBS on day 5. Tumor nodules were harvested on day 9 and RNA isolated for qPCR to determine viral replication (via A34R expression, left panel) and CXCL11 expression (right panel). Symbols: * stands for p < 0.05; ** p < 0.01; *** p < 0.001; and NS: not significant. Abbreviations: CM, culture medium; HPRT, hypoxanthine guanine phosphoribosyl transferase, a house-keeping gene.
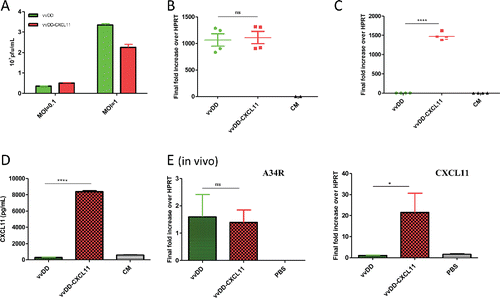
Figure 2. vvDD-CXCL11 treatment elicited antitumor effects in AB12-luc tumor model. AB12-luc cells (4 × 105) were inoculated i.p. into BALB/c mice and injected i.p. with PBS, vvDD or vvDD-CXCL11 (1 × 108 pfu/mouse) 5 d post tumor cell inoculation. (A). Tumor burden was measured by in vivo bioluminescence imaging on days 5 (D5) and 18 (D18) post tumor cell inoculation (n = 10 per group; only five mice per group are shown). One mouse (#4) in the PBS treated group has died of tumor burden on day 17, thus no image on D18. (B). Animal survival in AB12-luc tumor-bearing mice is presented using Kaplan–Meier survival curves. The curves represented data pooled from three independent experiments (n = 28 or more as indicated). p values are presented in the context of Results.
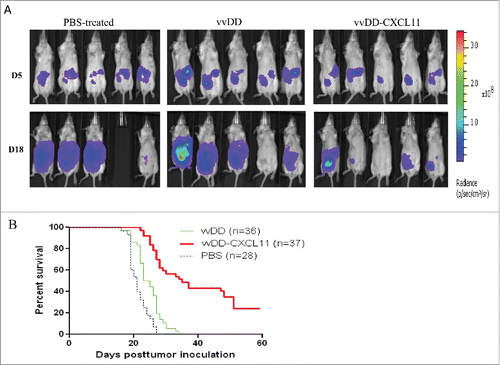
Figure 3. vvDD-CXCL11 treatment skewed the TME into a more favorable one for antitumor immunity. Tumor-bearing mice were treated as described in . Tumors nodules were harvested at day 9 post tumor inoculation, and were enzyme-digested and stained with PerCP-cy5.5-α-CD45 Ab and APC-α-CD8+ Ab (A, B). RNA was isolated for RT-qPCR to determine the expression of TGF-β (C), COX2 (D), CCL22 (E) and Granzyme B (F) in the TME. Symbols: * stands for p < 0.05; ** p < 0.01; *** p < 0.001; and NS: not significant. Abbreviations: RQ, relative quantity.
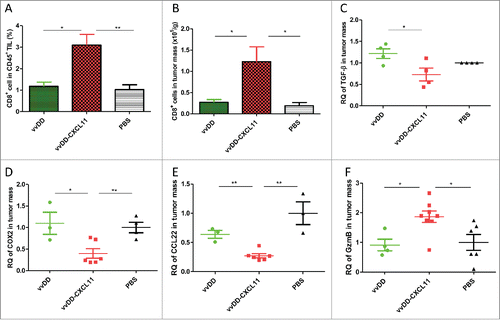
Figure 4. vvDD-CXCL11 treatment led to the accumulation of more CD8+ T cells in the spleen and mesenteric lymph nodes. Tumor-bearing mice were treated as described in . Mesenteric lymph nodes and spleens were harvested and single cells were stained with APC-α-CD8+ Ab day 20 post tumor cell inoculation. The percentage of CD8+ T cells in mesenteric lymph nodes and spleens are shown in (A) and (B). Whole splenocytes and absolute splenic CD8+ T cells are shown in (C) and (D).
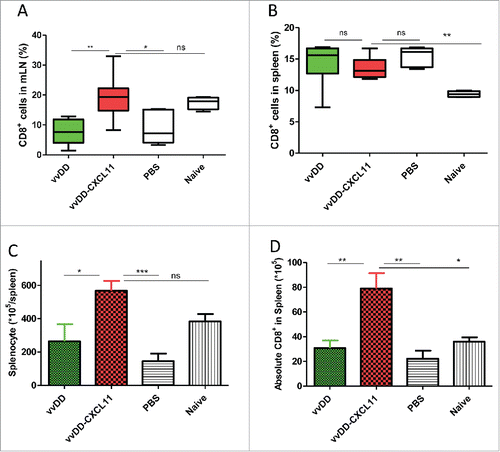
Figure 5. vvDD-CXCL11 elicited tumor-specific systemic immunity. Tumor-bearing BALB/c mice were treated as described in . Splenic CD8+ T cells (4 × 105 cells per assay) were isolated on day 20 post AB12-luc cell inoculation and restimulated with mitomycin C (MMC) treated AB12-luc (4 × 104) or control tumor CA51 cells (4 × 104) in the presence of 4,000-Rad-irradiated naive CD8− splenocytes (2 × 106) in 200 µL culture medium for 48 h. IFNγ in the culture supernatant was determined by ELISA (A). Splenocytes (3 × 107) from vvDD-CXCL11-cured mice 60 d post AB12-luc cell inoculation or PBS were adoptively transferred into naïve BALB/c mice and then challenged with AB12-luc (4 × 105) on the next day. Animal survival is presented using Kaplan-Meier survival curves (B). Symbol **** stands for p < 0.001.
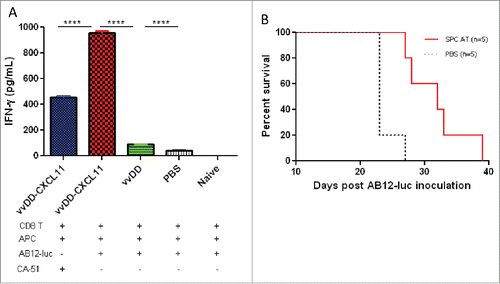
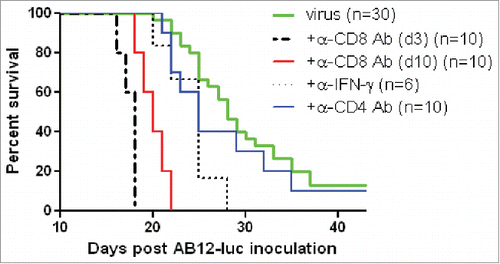
Figure 6. vvDD-CXCL11 elicited antitumor effects were dependent on CD8+ T cell and IFNγ. AB12-luc cancer cells (4 × 105) were inoculated i.p. into naive BALB/c mice on day 0 and tumor-bearing mice were injected i.p. with vvDD-CXCL11 (labeled as “virus”) at 1.0 × 108 pfu/mouse on day 5. For CD8+ depletion, anti-CD8+ Ab was injected i.p. on days 3, 4 and 5 or days 10, 11 and 12. For CD4+ depletion, anti-CD4+ Ab was injected i.p. on days 10, 15 and 19. For IFNγ neutralization, anti-IFNγ Ab was injected i.p. on days 10, 12, 14 and 16. Animal survival is presented using Kaplan–Meier survival analysis (n = 6 to 30 per group as shown). The p values between groups are presented in the main text in Results.