Figures & data
Figure 1. Peritoneal carcinomatosis and leukocyte attraction in the peritoneum. (A) Evaluation of the minimal tumorigenic dose by injection of murine colon cancer cells (MC-38) in the peritoneum of syngeneic mice. Results are expressed as a percentage of survival of n = 5 animals for each time point and dose of MC-38 cells injected in peritoneum. (B) Lesion weight and (C) number of peritoneal lesions at different time points. Results are expressed as mean ± SEM of data pooled from three independent experiments (n = 9 animals per each time point). (D) Representative Hematoxylin and Eosin (H&E) staining of neoplastic lesions obtained after intraperitoneal MC-38 cell injection. 20X magnification. (E) Cell counts in the peritoneal liquid (PL) at different time points after MC-38 cell injection. Results are expressed as mean ± SEM of three independent experiments (n = 9 animals per each time point); **p <0.005, *p <0.05, significantly different from cell counts in the PL of healthy mice (Unpaired T test with Welch correction). All mice received intraperitoneally the minimal tumorigenic dose of MC-38 (2 × 104 cells). (F) Representative dot plot of PL cells retrieved 21 d after intraperitoneal GFP+MC-38 cell injection (5 × 104cells per mouse). Physical parameters (Forward Scatter, FSC-A, x-axis vs. Side Scatter, SSC-A, y-axis, left) and fluorescence analysis (leukocyte CD45 expression, x-axis vs. GFP MC-38 y-axis, right). n = 10 animals for two independent experiments.
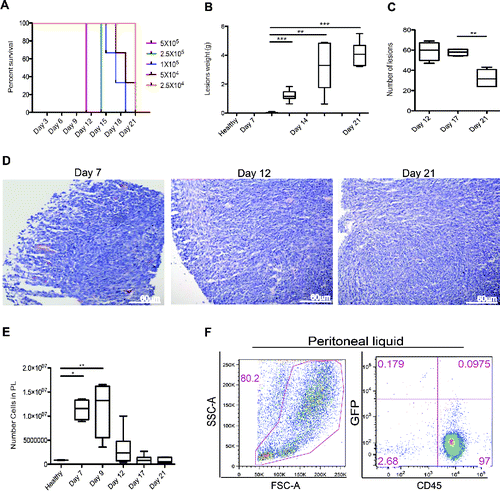
Table 1. Characterization of tumor-infiltrating leukocytes in peritoneal lesions and fluid.
Figure 2. Leukocyte infiltration and vascularization of the peritoneal lesions. (A) CD31-positive endothelial cells (red) in peritoneal lesions. Nuclei were counter-stained with DAPI (blue). 20X magnification. Vessels per Field of Vision (FOV) (B) mean vascular area (C) and ratio between the vascular area and the number of vessels (D) in peritoneal lesions retrieved at various times after intraperitoneal MC-38 cell injection. CD31+ cells were identified by digital image analysis.Citation15 Results are expressed as mean ± SEM of CD31+ cells/FOV, n = 15 animals for each time points for three independent experiment. (E) CD45+ leukocytes were revealed by immunohistochemistry in neoplastic lesions collected at days 7, 12 or 21. n = 9 animals for each time point for three independent experiments. (F) Representative dot plot of cells retrieved after enzymatic digestion of neoplastic peritoneal lesions collected at day 21. Physical parameters (Forward Scatter, FSC-A, x-axis vs. Side Scatter, SSC-A, y-axis left) and fluorescence analysis (leukocyte CD45 expression, x-axis vs. GFP MC-38 y-axis, right). (G) Table shows the percentage of cells population that infiltrate the peritoneal tumor lesions. n = 10 animals for two independent experiments.
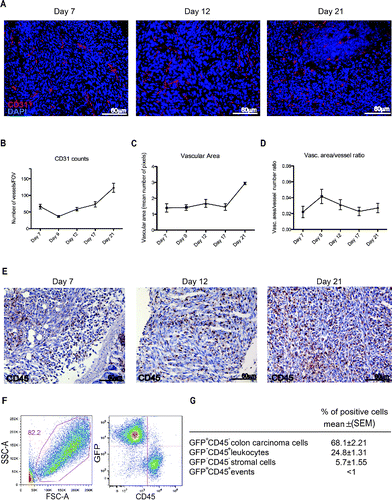
Figure 3. Recruitment of blood leukocytes into established peritoneal carcinomatosis lesions. 7 Tesla, T2*-weighted, MR images of abdomen of a representative mouse from tumor-bearing mice injected with syngeneic leukocytes labeled with SPIO particles (0.22 mg Fe/mL), at different time points (basal, 3 h, 24 h, panel A). At 24 h, the peripheral darkening of peritoneal lesions is strongly evident, without changes in signal intensity of the paravertebral muscle (negative control). A slight reduction of T2* relaxation time is detectable 3 h after SPIO-labeled leukocyte injection, which becomes stronger 24 h after injection (B, C). ROI based analysis of T2-maps: white color, skeletal muscle; black color, peritoneal lesions.
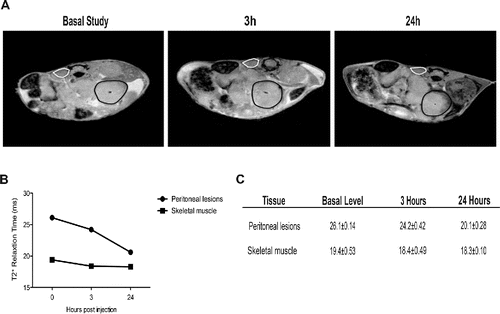
Figure 4. Growth and vascularization of peritoneal lesions upon macrophage depletion. (A) Schematic representation of macrophage depletion by treatment with liposomes containing Clodronate (Clodro-Lip) or saline (Sham-Lip). Treatment started 1 day after MC-38 carcinoma cell injection; mice were treated every other day until animal sacrifice (day 21). (B) Peritoneal lesion weight at sacrifice in the presence or the absence of macrophages was assessed and (C) Efficacy of the depletion was assessed by flow cytometry monitoring the percentage of F4/80+CD11b+ CD45+cells in the peritoneal liquid (D) immunohistochemistry showing infiltration of peritoneal lesions by CD68+ macrophages in Clodro-Lip (macrophage-depleted) and Sham-Lip (control) treated mice. (E) Number of infiltrating CD68+ macrophages per field of view in peritoneal lesions. (F) Mean vascular area in peritoneal lesions, calculated by digital image analysis. In panels (B), (C), (E), (F), data are expressed as mean ± SEM. n = 10 animals per group for two independent experiments. *p < 0.05; **p < 0.005; *p < 0.05; **p < 0.005 (Unpaired T test). (G) CD31+endothelial cells were revealed by immunohistochemistry in peritoneal lesions of Clodro-Lip and Sham-Lip (control) treated mice.
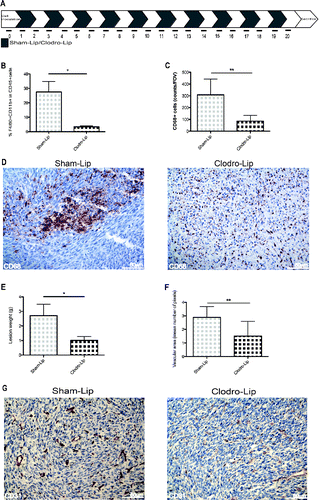
Figure 5. Colon cancer cell HMGB1 expression and biological activity. (A) Western Blot analysis of HMGB1 in murine colon carcinoma cells (MC-38); H-2b mouse lymphoma cells (RMA); mouse mammary adenocarcinoma cell line (TS/A); wild-type mouse Embryonic Fibroblast cells (MEF-wt) and Hmgb1 Knock Out Murine Embryonic Fibroblast cells (MEF-ko); murine colon adenocarcinoma cell lines (CT-26; C-26) and human colon adenocarcinoma cell lines (HT-29; Caco-2; HCT8; SW 620; WiDr). GAPDH was used as loading control. (B) HMGB1 expression (red) was revealed by immunofuorescence in living or freezed-thawed necrotic (F/T) MC-38 cells. Nuclei were counterstained with HOECHST (blue). (C) Murine bone-marrow-derived macrophage migration was assessed in a Boyden Chamber assay in response to conditioned media (CM) of living or necrotic (F/T) MC-38 cells, in the presence or the absence of HMGB1, anti-RAGE antibodies, Box A, anti-HMGB1 antibodies, and (E) the CXCR4 antagonist, AMD3100 as indicated. *p < 0.05; **p < 0.005; ***p < 0.001; significantly different from control (Unpaired T test).
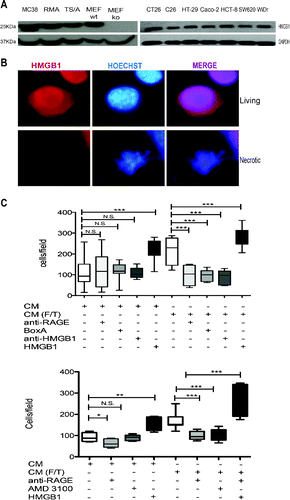
Figure 6. Exogenous HMGB1 promotes leukocyte recruitment into the peritoneal fluid and supports tumor growth. (A) Schematic representation of mice treatment with HMGB1. Treatment (1 µg/mouse) started 2 d after MC-38 carcinoma cell injection. Mice were treated every other day until animal sacrifice (day 15). (B) Tumor growth was monitored by [18F]FDG scans of HMGB1- or PBS-treated mice. Coronal (a) and transaxial (b) view of reconstructed images at the level of tumor location (dashed white line) are reported. Sites of [18F]FDG accumulation are indicated with arrows. Images were presented with the same scale and corrected for injected dose and animal weight. (C) Representative pictures of abdomens of HMGB1- or PBS-treated mice. Arrows indicate neoplastic lesions. (D) Volume of neoplastic lesions of HMGB1- or PBS-treated mice measured at PET (cm3, y-axis). Results are expressed as mean ± SEM. n = 5 per PBS- and n = 4 per HMGB1-treated groups, two independent experiments. (E) Weight of neoplastic lesions of HMGB1- or PBS-treated mice retrieved at sacrifice. Results are expressed as mean ± SEM of four independent experiments, n = 20. (F) CD68+ macrophages per Field of Vision (FOV) were identified by immunohistochemistry (y-axis) in peritoneal lesions of HMGB1- or PBS- treated mice retrieved at sacrifice (x-axis). Results are expressed as mean ± SEM of CD68+ cells/FOV. n = 10 animals per group, two independent experiments. (F) The mean vascular area (y-axis) was calculated in peritoneal lesions of HMGB1- or PBS-treated mice retrieved at sacrifice (x-axis) by digital image analysis. Data are expressed as mean ± SEM of results obtained. n = 10 animals per group, two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.001, significantly different from control (Unpaired T test).
![Figure 6. Exogenous HMGB1 promotes leukocyte recruitment into the peritoneal fluid and supports tumor growth. (A) Schematic representation of mice treatment with HMGB1. Treatment (1 µg/mouse) started 2 d after MC-38 carcinoma cell injection. Mice were treated every other day until animal sacrifice (day 15). (B) Tumor growth was monitored by [18F]FDG scans of HMGB1- or PBS-treated mice. Coronal (a) and transaxial (b) view of reconstructed images at the level of tumor location (dashed white line) are reported. Sites of [18F]FDG accumulation are indicated with arrows. Images were presented with the same scale and corrected for injected dose and animal weight. (C) Representative pictures of abdomens of HMGB1- or PBS-treated mice. Arrows indicate neoplastic lesions. (D) Volume of neoplastic lesions of HMGB1- or PBS-treated mice measured at PET (cm3, y-axis). Results are expressed as mean ± SEM. n = 5 per PBS- and n = 4 per HMGB1-treated groups, two independent experiments. (E) Weight of neoplastic lesions of HMGB1- or PBS-treated mice retrieved at sacrifice. Results are expressed as mean ± SEM of four independent experiments, n = 20. (F) CD68+ macrophages per Field of Vision (FOV) were identified by immunohistochemistry (y-axis) in peritoneal lesions of HMGB1- or PBS- treated mice retrieved at sacrifice (x-axis). Results are expressed as mean ± SEM of CD68+ cells/FOV. n = 10 animals per group, two independent experiments. (F) The mean vascular area (y-axis) was calculated in peritoneal lesions of HMGB1- or PBS-treated mice retrieved at sacrifice (x-axis) by digital image analysis. Data are expressed as mean ± SEM of results obtained. n = 10 animals per group, two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.001, significantly different from control (Unpaired T test).](/cms/asset/49a8524a-2303-468f-8dc5-8af69ca81b43/koni_a_1122860_f0006_oc.gif)
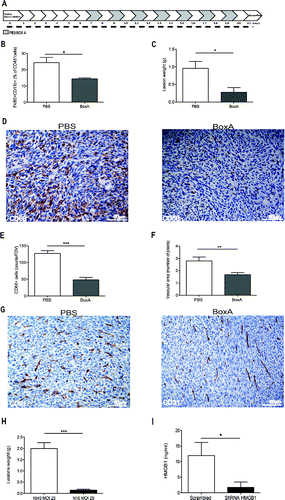
Figure 7. HMGB1 blockade reduces peritoneal carcinomatosis. (A) Schematic representation of mice treatment with BoxA. Treatment (300 µg/mouse) started 7 d after MC-38 carcinoma cell injection. Mice were treated every other day until suppression (day 21). Two independent experiments, n = 10 animals per group. (B) The percentage of F4/80+CD11b+ CD45+ cells in the peritoneal liquid of BoxA- or PBS-treated mice was assessed by flow cytometry and (C) peritoneal lesion weight at sacrifice of BoxA- or PBS-treated mice. (D) CD68+ macrophages in peritoneal lesions from BoxA- and PBS-treated mice. (E) Number of CD68+ macrophages/FOV in peritoneal lesions of BoxA- and PBS-treated mice. (F) Mean vascular area in peritoneal lesions, evaluated by digital image analysis. G: CD31+ endothelial cells in peritoneal lesions of BoxA- and PBS-treated mice. (H, I) Lesion weight (H) and the amount of HMGB1 in the peritoneal fluid (I) of mice transplanted with colon carcinoma cells expressing HMGB1 shRNA or LacZ a-miR LV-transduced were determined at the time of sacrifice (21 d). Three independent experiments, n = 15 animals per group. In panels (B), (C), (E), (F), (H), (I), data are expressed as mean ± SEM. *p < 0.05; **p < 0.005; ***p < 0.001, significantly different from control mice (PBS-treated mice in (B)–(G), mice injected with LacZ a-miR LV-transduced MC-38 colon carcinoma cells in (H) and (I).