Figures & data
Figure 1. NK cells express the long (α) and short (β) isoforms of myosin 18A. (A) Biotinylated YT2C2 leukemic cell lines were lysed and immunoprecipitated with DY12 mAb or control IgG. After migration on SDS-PAGE and transfer to nitrocellulose, the immunoprecipitated proteins were revealed with HRP-conjugated streptavidin and an ECL detection system. (B) Amino acid sequence of the 220–240 kDa protein immunoprecipitated by DY12 mAb as determined by mass spectrometry (MS) analysis. Underlined are the sequences common to that of myosin 18A. (C) YT2C2 cell lysates were immunoprecipitated using DY12 mAb or IgG control isotype. Immunoprecipitates were further subjected to immunoblotting using polyclonal anti-myosin 18A antibodies. Positions of the α (230 kDa) and β (190 kDa) isoforms of myosin 18A are indicated. (D) Fresh and healthy lung tissue from a human subject was lysed and extracts were immunoprecipitated with DY12 or control IgG mAb. Immunoprecipitates were immunoblotted using polyclonal anti-myosin 18A antibodies followed by HRP-conjugated goat anti-rabbit antibodies. The proteins immunoprecipitated by DY12 mAb corresponded to the α (230 kDa) and β (190 kDa) isoforms of myosin 18A.
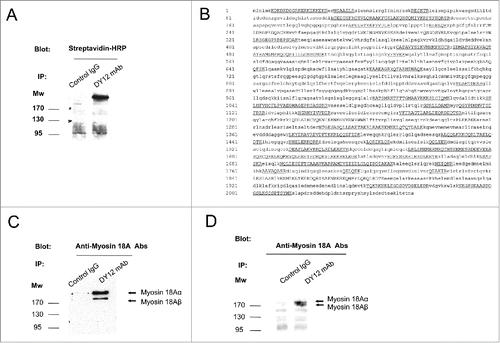
Figure 2. Myosin 18A/CD245 expression at the cell surface of peripheral blood mononuclear cells is constitutive and increased by activation. (A) PBMC from healthy donors were isolated and subjected to flow cytometry analysis using anti-CD245 DY12 mAb plus PE-coupled goat anti-mouse IgG antibodies and fluorochrome-conjugated, anti-CD3, -CD4, -CD8, -CD20, -CD56, -γδ TCR, or -CCR7 mAbs. (B) Flow cytometry analysis of CD245 expression on the lymphocytes and monocytes gates was assessed using DY12 mAb as in (A) Representative results from one experiment out of two are shown. (C–E) PBMC were stimulated for 72 h with IL-2 (100 IU/mL) (C), immobilized anti-CD3 mAb (D) or soluble anti-IgM antibodies (E) Expression of CD245/myosin 18A on NK, T and B cells was assessed using DY12 mAb as described in (A) and anti-CD56 (C), -CD3 (D) or CD19 (E) mAb, respectively. For each type of activation, representative results from one experiment out of two are shown.
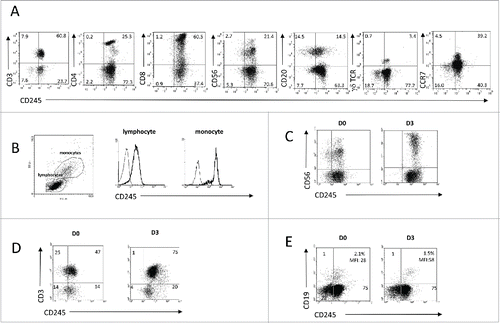
Figure 3. The engagement of myosin 18A/CD245 with DY12 mAb increases NK cell cytotoxicity. (A) Freshly isolated PB-NK lymphocytes from healthy donors were assessed for CD245 expression by immunohistochemistry using the DY12 mAb. Anti-granzyme B antibodies were used as positive control. (B) CD245-induced reverse cytotoxicity toward the P815 mastocytoma cell line. Cytotoxicity assays were performed according to a standard 51Cr-release method. Effector cells were left untreated (upper panels) or IL-2-activated (lower panels) prior to contact with the target cells. The target cells P815 were pre-incubated with DY12 or control mAb alone or together with an anti-CD335 (NKp46) or -CD337 (NKp30) mAb at 10 μg/mL. Assays were performed in triplicate at various E/T cell ratios, as indicated. Results are shown as mean percentages ± SD. Statistics were calculated using the ANOVA test, *p < 0.05, **p < 0.01, ***p < 0.001.
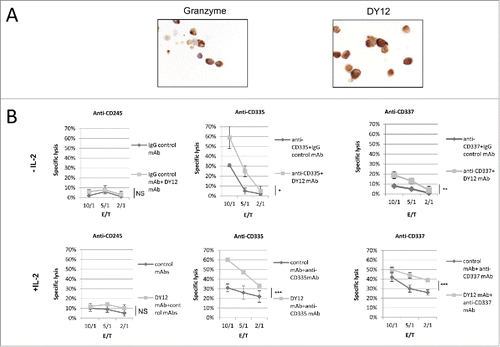
Figure 4. Engagement of CD245 with DY12 mAb or its physiological ligand SP-A increases the NK lymphocytes lymphokine-activated killer activity toward EBV-infected B cells. Cytotoxicity assays were performed according to a standard 51Cr-release method. Effector cells were untreated (A and C) or IL-2 activated (B and D) PB-NK lymphocytes from healthy donors and target cell was an Epstein-Barr Virus (EBV)-infected B cell line. (A and B) Target cells were preincubated with DY12 control F(ab')2 or control F(ab')2 mAb fragment at 10 μg/mL prior to contact with the NK cells. (C and D) The assay was performed in medium alone or the presence of recombinant SP-A at 100 ng/mL as indicated. Assays were performed in triplicate at various E/T cell ratios, as indicated Results are shown as mean percentages of specific lysis ± SD. Statistics were calculated using the ANOVA test, *p < 0.05.
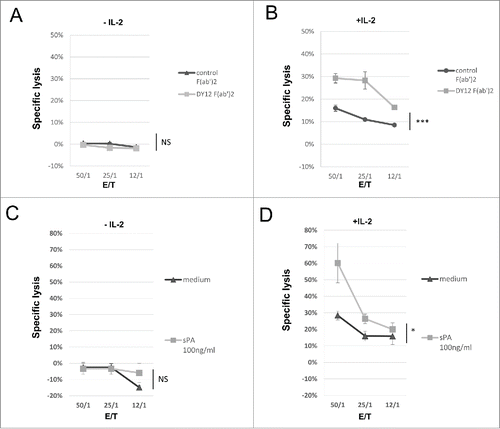
Figure 5. CD245-enhanced NK cell degranulation in the presence of 4-1BBL/CD137L-expressing target cells is 4-1BB/CD137-dependent. (A) The expression of the activating NK cell receptors NKp30, NKp44, NKp46 and NKG2D and of CD137 was monitored by flow cytometry on human NK cells triggered with an isotype control or DY12 mAb. FMO was used to determine positivity thresholds. The NK cells from five healthy donors were tested. Shown are the mean percentages ± SD of positive cells for each marker. Statistics were calculated with the Mann–Whitney U-test, **p < 0.01. (B) Raji cells were assessed for CD137L expression by flow cytometry. Unstained control was used to determine the positivity threshold. (C) PB-NK lymphocytes from healthy donors (n = 2) were incubated with DY12 or control IgG mAb (10 μg/mL), followed by cross-linking with rabbit anti-mouse IgG antibodies. The target cells were then added with or without anti-CD137L antibodies (10 μg/mL). CD107a expression was measured on CD3−CD56+ NK cells by flow cytometry. Results shown are mean percentages ± SD of CD107a MFI. Statistics were calculated using the ANOVA test, *p < 0.05.
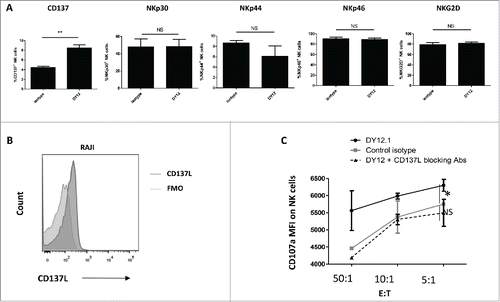
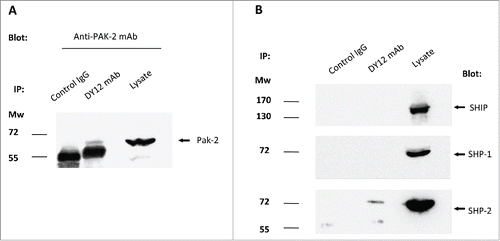
Figure 6. Myosin 18A co-precipitates with PAK-2 and SHP-2, two key signal transducers in NK cell activation and degranulation processes. Immunoprecipitates were performed on YT2C2 cell lysates with DY12 or control IgG1 mAb and subjected to electrophoresis and immunoblotting using anti-PAK2 (A), SHIP, SHP-1 or SHP-2 (B) antibodies. Total YT2C2 cell lysates were used as positive controls.