Figures & data
Figure 1. Mouse cancer cells with highly metastatic and aggressive phenotype express Cripto-1. (A) tumor cell lysates from lines on B6 background, RetV, MCA205, B16F10 and B16F1 were checked by protein gel blot for mouse Cripto-1 expression and compared relatively to β-actin. The D2F2 mouse Cripto-1 transfectant (D2F2mCR) was included as positive control. (B) B16F10 tumor cells were grown on low attachment plates for one to three passages (P1, P2 and P3) to enrich for tumor initiating cells and cell lysates were generated from each passage. (C) Expression levels of mouse cripto-1 were assessed from healthy mouse tissue from the GSE1133 data set and represented sorted with highest expression to the left.
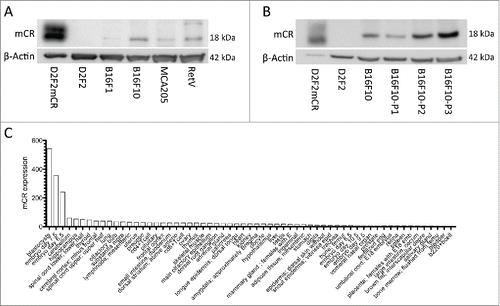
Figure 2. Mouse cripto-1 peptides bind to H2-Kb in vitro and in silico. (A) RMA-s cells were used to measure surface stabilization of H2-Kb using an overlapping 15-mer peptides library. H2-Kb was analyzed by flow cytometery after loading with 100 µg/mL of peptide for 2 h, with negative controls (un-stained, filled gray, and un-load, green), positive controls (OVA and TRP2, red) as well as the peptides mCR46-60, mCR16-30, mCR1-15 (solid black). (B) 9mers within Cripto-1 were identified using in silico prediction analysis with NetMHC.
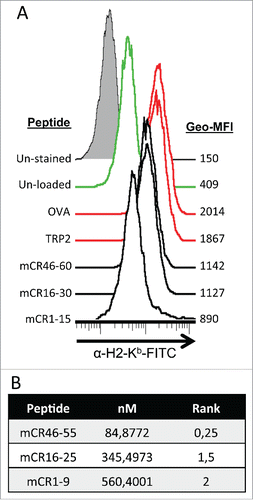
Figure 3. Vaccination with pmCR elicits an epitope specific functional CD8+ T cell response. (A) Peripheral blood lymphocytes from vaccinated mice (n = 9) were stimulated with mCR16-25 specifically for 8 h prior to ICS and significant increase in IFNγ/TNF-α producing cells were found; *** p < 0.001 using Mann–Whitney t-test and a representative dot-plot is shown. (B) Splenocytes from naïve mice were harvested and labeled with lo and hi CFSE prior to loading with OVA and mCR16-25, respectively, and transferred into pmCR or pVAX vaccinated mice. Specific killing was analyzed by FACS after 20 h. mCR16-25 specific killing of splenocytes in vivo was found to be significantly greater in pmCR vaccinated mice; *** p < 0.001 using Student's t-test. (C) CD8+ T cells were isolated using MACs bead positive selection from B6 (n = 5) mice vaccinated with either pmCR or pVAX and stimulated with CR expressing tumor cell line B16F10, mCR16-25 peptide, OVA peptide or with T cells alone; ** p < 0.01 using Mann–Whitney t-test.
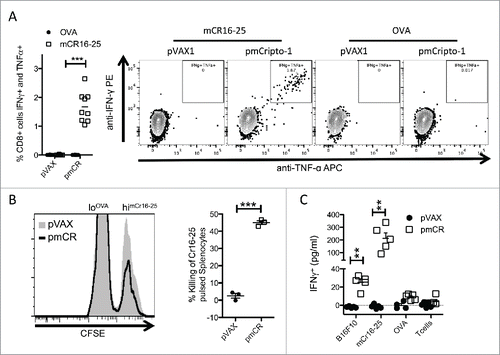
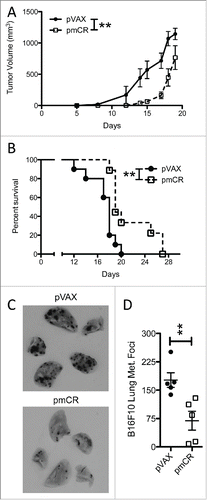
Figure 4. Vaccination against mouse Cripto-1 increases survival and reduces metastatic lung burden in B6 mice challenged with mouse Cripto-1 expressing B16F10. (A) Tumor growth was compared in B6 mice (n = 10 per group) vaccinated prophylactically with either pmCR or pVAX with two doses of 20 µg plasmid DNA delivered i.d. with electroporation prior to s.c. challenge with 5×104 B16F10 cells with 2-way ANOVA test; **p < 0.01. (B) Survival of B6 mice vaccinated with pmCR or pVAX was compared after challenge with s.c. B16F10 with Mantel–Cox test; **p < 0.01. The results shown here are representative of two experiments. (C) Representative photos of B16F10 metastatic lung burden in vaccinated mice harvested 14 d post i.v. challenge of 2×105 tumor cells. D, reduction in lung metastasis was evaluated by enumerating foci of tumors in lungs of B16F10 i.v. challenged mice and compared with non-parametric t-test; *p < 0.05. The results shown here are representative of three experiments.