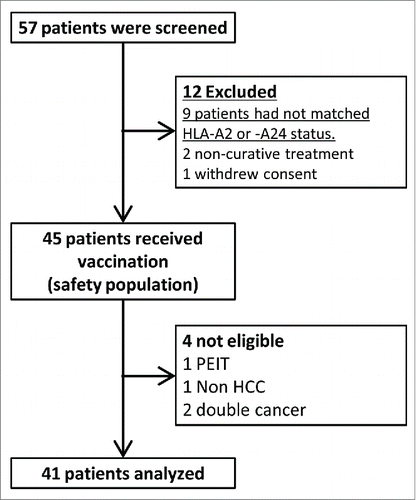
Figures & data
Table 1. Patient characteristics.
Table 2. Adverse events.
Figure 2. Kaplan–Meier curves for recurrence-free survival and overall survival in 41 patients who received vaccinations in conjunction with surgery or radiofrequency ablation.
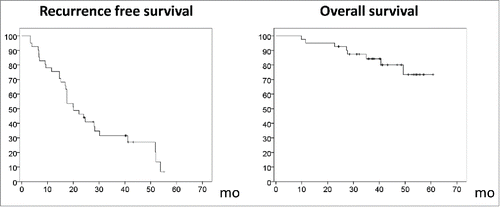
Figure 3. Cytotoxic T lymphocyte (CTL) analysis in peripheral blood mononuclear cells (PBMCs) and recurrent tumors. (A) Immunohistochemical staining for glypican-3 (GPC3) showed positivity in the primary tumor. The recurrent tumor appeared to lack GPC3 expression. Change in the expression of GPC3 in cases 42 and 53 after GPC3 peptide vaccination. Magnification = 200X. (B) Ex vivo interferonγ (IFNγ) ELISPOT assays for GPC3 in 5 × 105 peripheral blood mononuclear cells were performed before and after vaccination. Δ spot number indicates the number of GPC3-peptide-specific CTLs. The number of IFNγ-positive spots increased in the wells pre-incubated with GPC3 peptide.
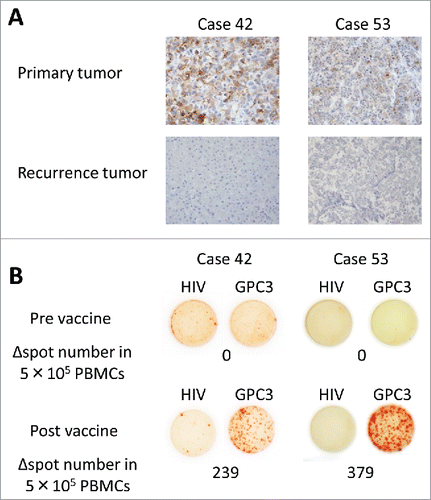
Figure 4. Kaplan–Meier curves for recurrence-free and overall survival. (A) Thirty-five patients treated with surgery and vaccination did not have significantly longer recurrence-free survival or overall survival rates than the 33 patients who underwent surgery only. (B) Among patients with glypican-3 (GPC3)-positive tumors, the recurrence rate was significantly lower in the 25 patients treated with surgery and vaccination compared to the 21 patients who underwent surgery only (24% vs. 48% and 52.4% vs. 61.9% at 1 and 2 y, respectively; p = 0.047, 0.387). The 25 patients treated with surgery and vaccination tended to have longer recurrence-free and overall survival rates compared to the 21 patients who underwent surgery only.
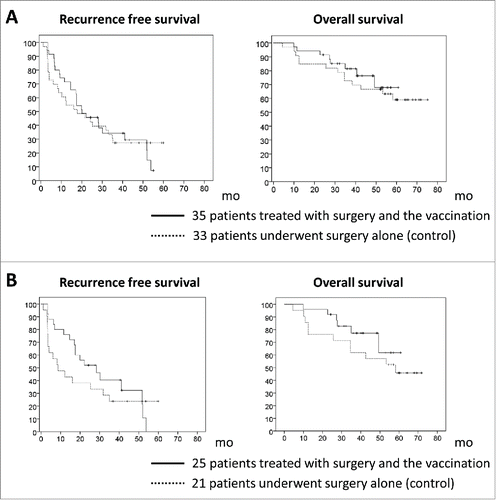
Table 3. Patient characteristics.