Figures & data
Figure 1. Immunophenotyping of Syrian hamster tumors. Percentage of CD4+ (black bar) and CD8+ (gray bar) cells in various Syrian hamster tumors processed into single-cell suspensions, stained with cross-reactive antibodies and analyzed by flow cytometry (50,000 events). Data representative of four tumors. Error bars, SE.
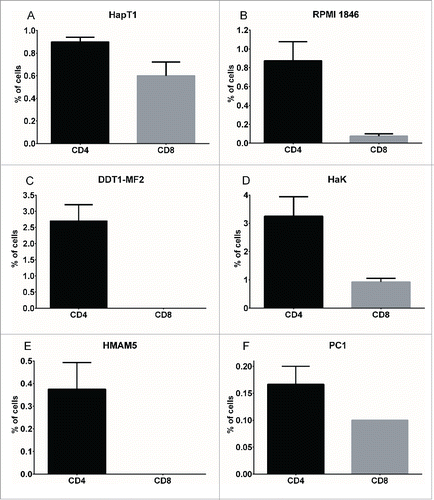
Figure 2. Lymphocyte composition of TIL cultures. Tumor fragments were cultured in recombinant human IL-2 (6,000 IU/mL) on 24-well plates. On Day 5, when TIL outgrowth became visible, the tumor fragment was removed and remaining TIL were analyzed for CD4+ and CD8+ by flow cytometry. On Day 10, individual wells with TIL outgrowth was combined and the pooled sample was analyzed for CD4+ and CD8+. Data representative of four fragments. Error bars, SE.
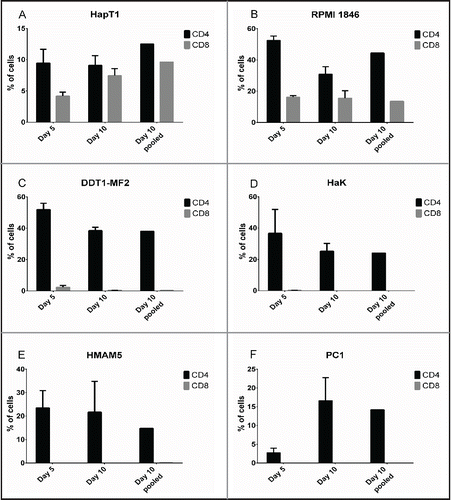
Figure 3. Cytolytic activity of TIL ex vivo. Pooled Day 10 TIL were co-cultured with either autologous cancer cells, non-related hamster cells (DDT1-MF2 or equivalent) or human cells (A549) with different effector-to-target ratios (E/T). Target cell viability was determined 24 h later by MTS.
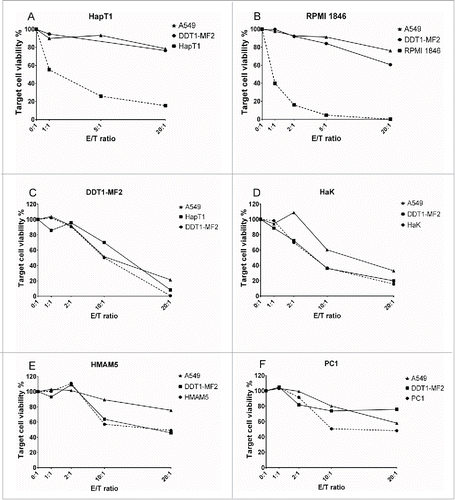
Figure 4. Effect of MHC Class I blocking on cytolytic activity of TIL. (a) RPMI 1846 and (b) HapT1 target cells were pre-incubated for 2 h with anti-MHC Class I antibody (50 µg/mL), isotype control (50 µg/mL) or left untreated (no inhibition) before adding TIL. Target cell viability was determined 24 h later by MTS. Error bars, SE. *p < 0.05, ns = not significant.
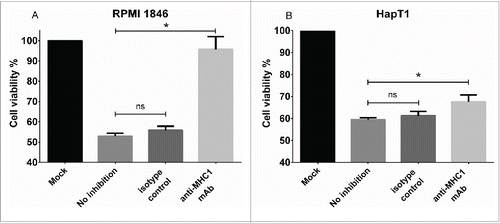
Figure 5. Improved in vitro cell killing with TIL and Ad5-D24 combination. HapT1 cells were infected with Ad5-D24 (100 VP/cell) for 3 d before adding HapT1 TIL. Target cell viability was determined 24 h after TIL addition. Error bars, SE. ****p < 0.0001.
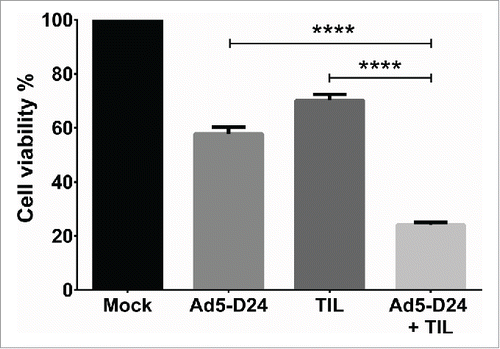
Figure 6. Combination treatment of established HapT1 tumors with TIL and Ad5-D24. Subcutaneous HapT1 tumors were allowed to develop on both flanks of Syrian hamsters (5 × 106 cells per flank) before reaching 6 mm in diameter. On Days 1 and 8, Ad5-D24 (1 × 107 VP/tumor) was injected intratumorally. On Day 2, HapT1 TIL (1.5 × 106 TIL/tumor) were administered intratumorally. Error bars, SE. *p < 0.05, **p < 0.01.
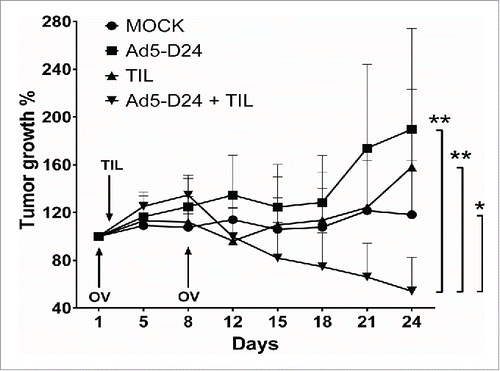
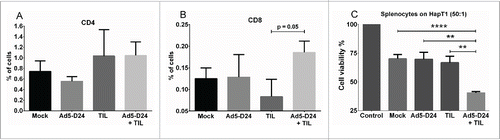
Figure 7. T-cell infiltration and splenocyte cell killing activity following treatment with TIL and Ad5-D24. (a) CD4+ and (b) CD8+ T cells were analyzed from Day 24 tumors by flow cytometry. (c) Splenocytes (collected from hamsters sacrificed on Day 24) were co-cultured with HapT1 cells at the effector-to-target ratio of 50:1. Target cell viability was determined 24 h later by MTS. Error bars, SE. **p < 0.01, ****p < 0.0001.