Figures & data
Figure 1. Immunohistochemical staining of MDM2, c-Myc in Lung cancer tissue and adjacent normal tissue slides. Tissue slides were stained with monoclonal anti-MDM2 antibody (A, B, C) and anti-c-Myc antibody (D, E, F), respectively. (A) Negative stain pattern of MDM2 in representative adjacent normal tissue (×400 magnification). (B and C) Positive stain pattern of MDM2 in representative lung cancer tissue (×100 and ×400 magnification). (D) Negative stain pattern of c-Myc in representative adjacent normal tissue (×400 magnification). (E and F) Positive stain pattern of c-Myc in representative lung cancer tissue (×100 and ×400 magnification).
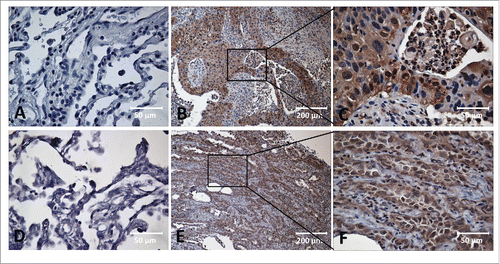
Table 1. MDM2 and c-Myc Protein Expression in 43 pair-wise lung cancer tissues.
Table 2. Frequency of autoantibody against MDM2 and c-Myc in human sera by ELISA.
Figure 2. Spearman correlation of MDM2 and c-Myc IHC scores and serum levels of autoantibodies against MDM2 and c-Myc. Spearman correlation analysis was used to explore the link between MDM2 and c-Myc IHC scores and serum autoantibodies against these two TAAs in 43 lung cancer cases with paired tissues and sera samples. The results show that MDM2 and c-Myc protein expression in lung cancer tissues are positively associated with serum autoantibodies against these two TAAs respectively. (rs = 0.360, p = 0.018 and rs = 0.526, p < 0.001, respectively).
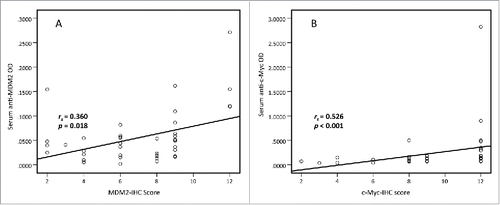
Figure 3. Serum anti-MDM2 autoantibodies in lung cancer and NHC. In , Y-axis represents OD value and X-axis represents serum samples (*p < 0.05; **p < 0.01). (A) Serum anti-MDM2 autoantibodies distribution in NHC (n = 44) and lung cancer patients (n = 50) (p = 0.001). (B) ROC curve analysis using serum anti-MDM2 autoantibodies for discriminating lung cancer patients from NHC in research group (AUC: 0.698, 95% CI: 0.592–0.805, p = 0.001). (C) Serum anti-MDM2 autoantibodies distribution in the validation group including NHC (n = 43) and lung cancer patients (n = 62) (p = 0.005). (D) ROC curve yielded by OD values of serum anti-MDM2 autoantibodies for discriminating lung cancer patients from NHC in the validation group (AUC: 0.777, 95% CI: 0.689–0.865, p < 0.001). (E) Serum anti-MDM2 autoantibodies distribution and in NHC and lung cancer patients with different TNM stage. (F) ROC curve yielded by OD values of anti-MDM2 autoantibodies in discriminating stage I lung cancer patients from NHC (AUC: 0.703; 95% CI: 0.5800–0.827, p = 0.003). (G) ROC curve yielded by OD values of anti-MDM2 autoantibodies in discriminating stage II lung cancer patients from NHC (AUC: 0.622; 95% CI: 0.432–0.812, p = 0.161). (H) ROC curve yielded by OD values of anti-MDM2 autoantibodies in discriminating stage I lung cancer patients from NHC (AUC: 0.866; 95% CI: 0.741–0.991, p = 0.004). Note: LC1: 50 lung cancer patients from research group. LC2: 62 lung cancer patients from validation group.
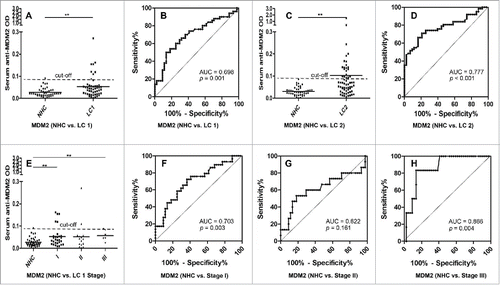
Figure 4. Serum anti-c-Myc autoantibodies in lung cancer patients and NHC. In , Y-axis represents OD value and X-axis represents serum samples (*p < 0.05; **p < 0.01). (A) Serum anti-c-Myc autoantibodies distribution in NHC (n = 44) and lung cancer patients (n = 50) (p = 0.049). (B) ROC curve analysis using serum anti-c-Myc autoantibodies for discriminating lung cancer patients from NHC in research group (AUC: 0.636, 95% CI: 0.524–0.747, p = 0.024). (C) Serum anti-c-Myc autoantibodies distribution in the validation group including NHC (n = 43) and lung cancer patients (n = 62) (p = 0.001). (D) ROC curve yielded by OD values of serum anti-c-Myc autoantibodies for discriminating lung cancer patients from NHC in the validation group (AUC: 0.815, 95% CI: 0.733–0.896, p < 0.001). (E) Serum anti-c-Myc autoantibodies distribution and in NHC and lung cancer patients with different TNM stage. (F) ROC curve yielded by OD values of anti-c-Myc autoantibodies in discriminating stage I lung cancer patients from NHC (AUC: 0.662; 95% CI: 0.532–0.791, p = 0.020). (G) ROC curve yielded by OD values of anti-c-Myc autoantibodies in discriminating stage II lung cancer patients from NHC (AUC: 0.567; 95% CI: 0.377–0.756, p = 0.444). (H) ROC curve yielded by OD values of anti-c-Myc autoantibodies in discriminating stage I lung cancer patients from NHC (AUC: 0.682; 95% CI: 0.439–0.924, p = 0.152). Note: LC1: 50 lung cancer patients from research group. LC2: 62 lung cancer patients from validation group.
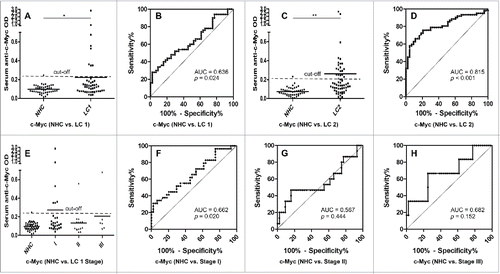
Table 3. Relationship between clinicopathological factors and autoantibodies against MDM2 and c-Myc in lung cancer patients.
Figure 5. Disease-free survival (DFS) of high- and low-serum anti-c-Myc autoantibodies levels in lung cancer patients from research group. 50 lung cancer patients from research group were divided into two subgroups by using the median OD value of serum anti-c-Myc autoantibodies, as were high- and low-serum anti-c-Myc autoantibodies expression subgroups respectively, and 25 lung cancer patients were included in each subgroup. Log-rank test showed that lung cancer patients with higher anti-c-Myc autoantibodies were associated with shortened DFS (χ2 = 4.172, p = 0.041).
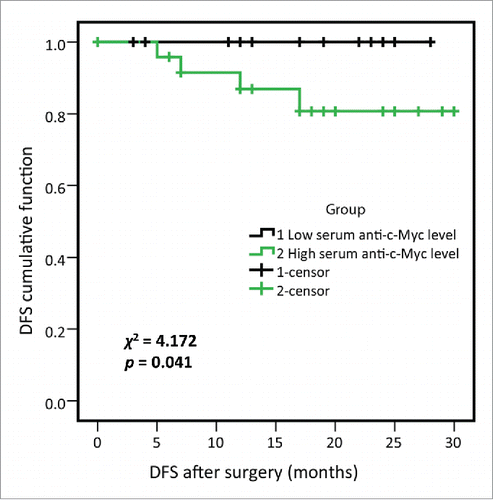
Table 4. Univariate and Multivariate analyses of OS/DFS (Cox proportional hazards regression model).
Figure 6. Western Blot results validated the results from ELISA. Western Blot analysis with representative sera from lung cancer patients and NHC. Lanes 1–10 (research group), Lanes 11, 12 (validation group), sera from lung cancer patients; Lanes 13–16, sera from NHC. Lanes 4, 5, 7 and 9 show immunoreactivity with both MDM2 and c-Myc recombinant proteins; Lanes 6, 10 and 13 show strong reactivity with c-Myc recombinant protein, while they show weak reactivity with MDM2; Lanes 1, 8 and 12 show strong reactivity with MDM2 recombinant protein, while they show weak reactivity with c-Myc; Lanes 2, 3, 11, 14, 15 and 16 show no reactions with MDM2 or c-Myc. The last two lanes are negative control and positive control of MDM2 and c-Myc, respectively. Note: MDM2 and c-Myc proteins transferred to the NC membrane were purified his-tag proteins.

