Figures & data
Table 1. Baseline patient characteristics.
Figure 1. Disease status of UM patients participating in the Pembrolizumab EAP. Treatment was administered at 3 weekly intervals with tumor assessments performed at baseline and every three cycles of treatment or as clinically indicated.
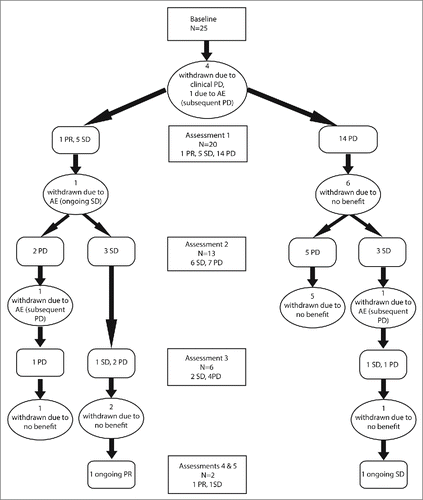
Table 2. Best radiological disease response to pembrolizumab; nine patients were alive, one with an ongoing partial response and two with stable disease at the time of writing hence median overall and progression free survival was not reached (NR) for several subgroups.
Figure 2. Kaplan–Meier plots of overall and progression free survival of UM patients treated with pembrolizumab at 2mg/kg as part of the expanded access program. (A) Curves for entire group, median OS not reached. (B–H) Curves stratified by best previous response to ipilimumab (B), site of disease progression at baseline (C–D), number of liver directed treatments received prior to enrolling to the EAP (E), number of previous systemic treatments (F), serum LDH (G) and ECOG PS (H).
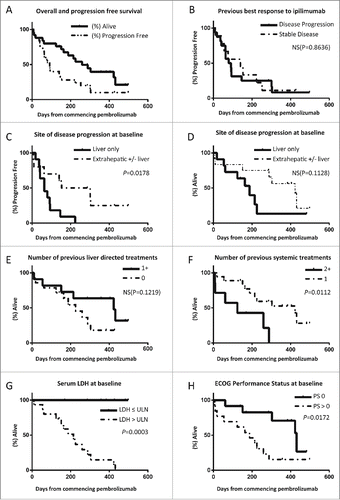
Figure 3. Plots demonstrating lead- in times from diagnosis of UM recurrence to commencing pembrolizumab stratified by (A) location of disease progression at time of commencing pembrolizumab, (B) number of liver directed treatments received prior to enrolling to the EAP and (C) number of previous systemic treatments.
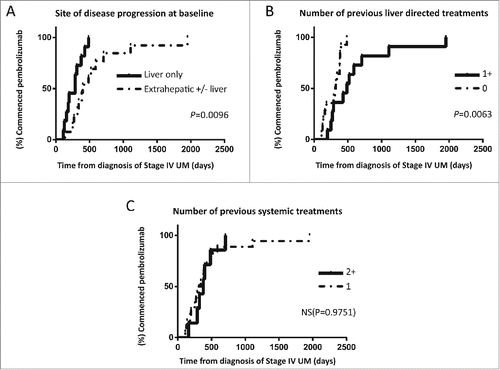
Table 3. Treatment related adverse events.
