Figures & data
Figure 1. Poly(I:C) induces BATF3-mediated WT1-C1498 tumor regression. WT1-C1498 tumor was implanted to wild-type or Batf3−/− mice (C57BL/6J) and Poly(I:C) was administered around tumor at day 5 and 12 after tumor implantation (A, C). The tumor volume was measured every 2 to 3 d. For depleting CTLs or NK cells, ascites containing anti-CD8α or anti-NK1.1 antibody was i.p. injected the day before Poly(I:C) therapy (B, D). WT1-C1498 tumor was harvested at day 19 as in panels A and C, and the proportion of tumor-infiltrating CD8+ T cells was evaluated by flow cytometer (E). Db126 peptide wrapped in DOTAP with or without Poly(I:C) were administered to tumor-bearing wild-type mice at day 5 and 12 after tumor implantation. At day 20, splenocytes were harvested and cultured in complete medium in the presence of Db126 (5 μg/mL) for 5 d. At day 25, the proportion of WT1-specific CD8+ T cells was evaluated (F). At day 21, splenocytes of Batf3−/− mice treated as in panel F were harvested. The cells were re-stimulated with Db126 and the proportion of WT1-specific CD8+ T cells was evaluated as in panel F (G). Arrows show the day of Poly(I:C) administration. Error bars show ± SEM; n = 3 to 4 per group. Student's t-test was performed to analyze statistical significance. *p < 0.05. ns; not significant. The results are the representatives of more than two independent experiments.
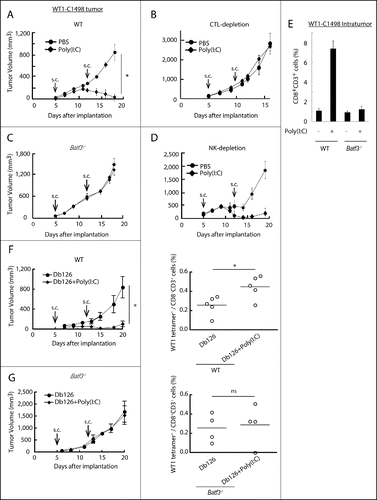
Figure 2. Combined administration of antigen and Poly(I:C) induces EG7 tumor regression. Wild-type mice were inoculated with EG7 tumor and had Poly(I:C) and OVA therapy day 5 after tumor implantation. At day 12, the proportion of tumor-infiltrating OVA-specific CD8+ T cells was evaluated (A). EG7 was implanted to wild-type mice or Batf3−/− mice and Poly(I:C) with or without OVA was administered around the tumor at day 7 and 14. PBS was used as control (B). At day 16, the proportions of OVA-specific CD8+ T cells in spleen were evaluated by flow cytometer (C). EG7-bearing wild-type mice had Poly(I:C) and OVA therapy at day 7, and at day 15, the proportion of tumor-infiltrating OVA-specific CD8+ T cells was evaluated (D). Error bars show ± SEM; n = 4 to 8 per group. Student's t-test (A, B) and Kluskal–Wallis test with Dunn's multiple comparison test (C, D) were performed to analyze statistical significance. *p < 0.05, ns; not significant. Three similar experiments were performed and the results are the representative one.
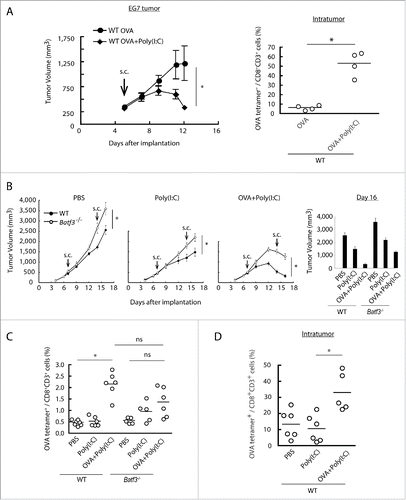
Figure 3. The cross-priming is partially reduced in Batf3−/− mice. Poly(I:C) and OVA were administered to wild-type and Batf3−/− mice with no tumor-loading. After 7 d, the proportion of OVA-specific CD8+ T cells in spleen was evaluated with tetramer by flow cytometer (A). CD8α+ DCs isolated from spleens of wild-type, Batf3−/− and Tlr3−/− mice were incubated with Poly(I:C) and OVA for 4 h and then co-cultured with CFSE-labeled OT-1 cells. After 60 h, the antigen-specific OT-1 proliferation was evaluated by diminution of CFSE (B) and IFNγ in the culture supernatant (C). Shadow histograms show wild-type specimen treated with PBS without OVA. Student's t-test was performed to analyze statistical significance. *p < 0.05. More than three similar experiments were performed and the results are the representative one.
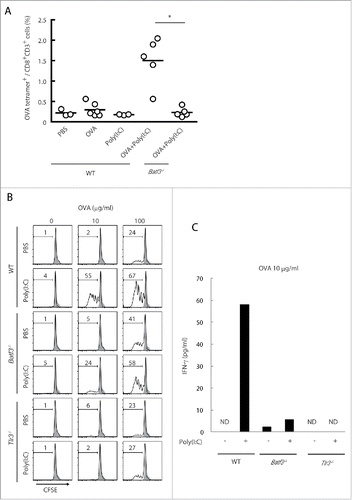
Figure 4. TLR3 and inducible IL-12 levels are decreased in CD8α+ DCs in Batf3−/− mice. CD8α+ DCs in spleen were isolated from wild-type and Batf3−/− mice and then the levels of mRNA were evaluated. The expression levels of the indicated genes were measured by qPCR (A). The level of TLR3 protein in CD8α+ DCs was assessed by flow cytometer (B). CD8α+ DCs were isolated from wild-type, Tlr3−/− and Batf3−/− mice, and stimulated with Poly(I:C). After 4 h, mRNA were collected and the expression levels of the indicated genes were evaluated (C). Poly(I:C) was s.c. administered to wild-type and Batf3−/− mice. The blood serum was collected at the times indicated and the amount of IL-12p40 was measure by ELISA (D). Poly(I:C) was s.c. administered to wild-type and Batf3−/− mice. 12 h later, splenocytes were harvested. Collected cells were cultured in the presence of Blefeldin A for 4 h. Then, the proportion of IL-12p35+ p40+ CD8α+ DCs (MHC class II+ CD11chi) was evaluated by flow cytometer (E). Error bars show ± SEM; n = 4 to 6 per group. Kluska–Wallis test with Dunn's multiple comparison test were performed to analyze statistical significance. *p < 0.05, ns; not significant (E). The results are the representatives of three independent experiments.
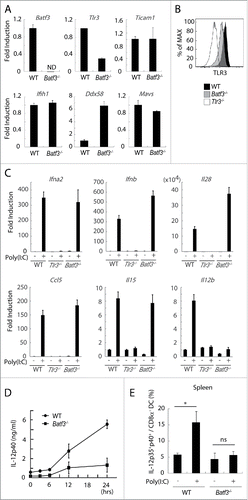
Figure 5. Poly(I:C)-induced CD8+ cell infiltration into tumor tissues. EG7 tumor-bearing mice had Poly(I:C) + OVA therapy on day 7 and 14, and tumor tissues were excised at day 16. The tumor sections were stained with hematoxylin and eosin (A) or APC-anti CD8α antibody and DAPI (B, C). The confocal microscopy images (×20 magnification) of a margin and a center of the tumor isolated from wild-type mice (Upper panels) or Batf3−/− mice (Lower panels) are shown (Red; CD8α, Gray; DAPI) (B). The numbers of CD8α+ cells in 0.2 mm2 of tissue sections were counted (C). Tumor-bearing mice had Poly(I:C) and OVA therapy at day 9 and tumor tissues were excised at day 15. Tumor sections were stained with APC-anti-CD8α , PE-anti-TCR Vα2 antibody and DAPI (D). The confocal microscopy images are shown (Red; CD8α, Green; TCR Vα2, Gray; DAPI) (Left panel). The numbers of CD8α+ TCR Vα2+ cells in 0.2 mm2 of the tissue sections were counted (Right panel) (D). The proportion of tumor-infiltrating CD8α+ CD3+ cells was evaluated by flow cytometer (E). The results are the representatives of each group (B, C, D). Error bars show ± SEM; n = 5 per group (E). Kluskal–Wallis test with Dunn's multiple comparison test (C, E) and Student's t-test (D) were performed to analyze statistical significance. *p < 0.05, ns; not significant.
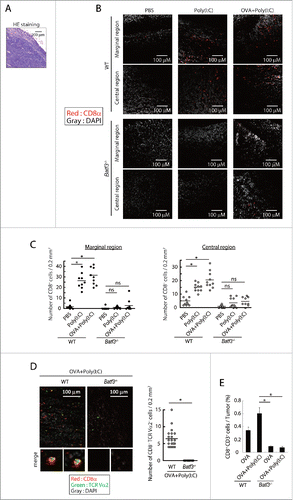
Figure 6. Poly(I:C) changes intratumoral gene expression patterns and IL-12 production in wild-type mice but not in Batf3−/− mice. EG7 tumor-bearing mice were challenged with Poly(I:C) and OVA at day 9 and 14 after tumor implantation. At day 15, tumor tissues were harvested and the levels of mRNA were evaluated (A, D). The quantity of the IL-12p40 and CXCL10 proteins in the fixed volume of tumor tissues was also evaluated by ELISA (B, C). The ratios of tumor-infiltrating CD8α+ DC and CD103+ DC subsets were evaluated by flow cytometer (E). Error bars show ± SEM; n = 4–7 per group. One-way analysis of variance (ANOVA) with Bonferroni's test was performed to analyze statistical significance (E). *p < 0.05, ns; not significant. The results are one of the two independent experiments. In panel B, one representative of each group is shown.
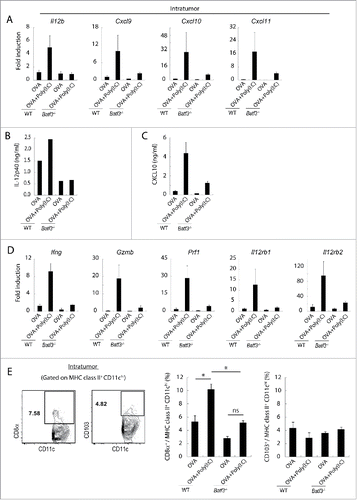
Figure 7. BATF3 plays a key role in CD8α+ DCs for IL-12 production. CD8α+ DCs were isolated from wild-type (Left panel) or Batf3−/− mice (Right panel) and i.v. transferred to WT1-C1498 tumor-bearing Batf3−/− mice at day 4 and 11 after tumor implantation. PBS or Poly(I:C) were administered to the recipient mice at day 5 and 12 (A). WT1-C1498 was implanted to Batf3−/− mice and the mice were treated with Poly(I:C) alone or Poly(I:C) and IL-12p70 (Left panel), PBS or IL-12p70 (Right panel) at day 5 and 12 (B). At day 13, tumor tissues were harvested and the proportion of tumor-infiltrating CD8+ T cells was evaluated by flow cytometer (C). The spleen was also harvested and the proportion of CD8α+ DCs was evaluated (D). Error bars show ± SEM; n = 3 to 5 per group. Kluskal–Wallis test with Dunn's multiple comparison test (the left B) and Student's t-test was performed (A, the right B) to analyze statistical significance. *p < 0.05. Two similar experiments were additionally performed and supported the results shown.
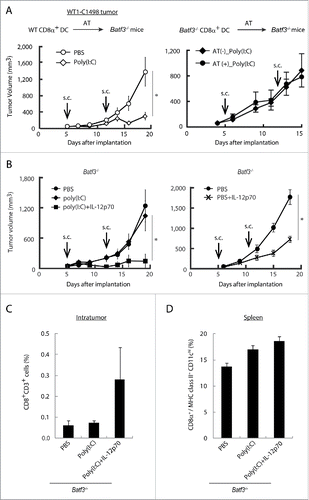
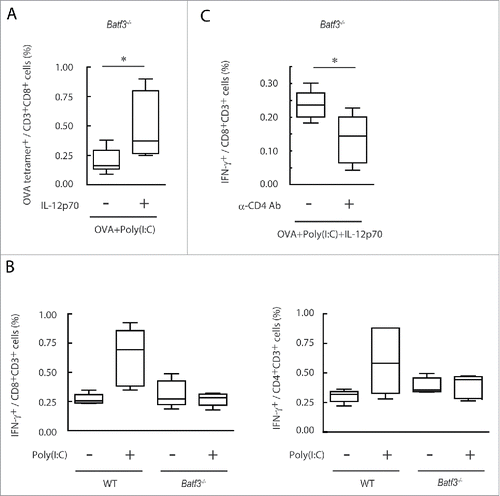
Figure 8. IL-12p70 induces CTL activation and CD4+ T cell assist it in Batf3−/− mice. Poly(I:C) and OVA (1 mg) with or without IL-12p70 were i.p. administered to Batf3−/− mice. 7 d later, splenocytes were harvested and the proportion of OVA-tetramer+ CD8+ T cells was evaluated (A). Poly(I:C) and OVA (60 μg) were administered to wild-type or Batf3−/− mice at day 0 and 7. At day 14, the proportion of IFNγ+ CD8+ T and CD4+ T cells in spleen were evaluated (B). Acsites containing anti-CD4 antibody were injected to Batf3−/− mice at day 0 and 7. 24 h later, Poly(I:C) and OVA (250 μg) with or without IL-12p70 were administered to Batf3−/− mice. At day 15, the proportion of IFNγ+ CD8+ T cells in spleen was evaluated (C). Error bars show ± SEM; n = 4 to 5 per group. Student's t-test (A, C) and Kluskal–Wallis test with Dunn's multiple comparison test (B) were performed to analyze statistical significance. *p < 0.05. Two similar experiments were performed and the results were supported.