Figures & data
Figure 1. Glioblastoma (GBM) vaccines and their interaction with immunity. (1) Rindopepimut (RINTEGA), a synthetic peptide vaccine targeted at the EGFRvIII mutation, and HSPPC-96 (Prophage), an autologous-derived complex consisting of heat shock proteins complexed with GBM antigens, are intradermally injected for uptake by resident dendritic cells (DC). (2) The vaccines are co-administered with adjuvants, such as GM-CSF and keyhole limpet hemocyanin (with respect to RINTEGA) for stimulating DC uptake, antigen processing and upregulation of costimulatory molecules. (3) Peripheral blood mononuclear cells can be isolated from patient blood (not shown), expanded in culture, primed with autologous patient-derived GBM tumor lysate, followed by intravenous transfer back into the patient, bypassing the need for dermal injection of tumor antigens. Regardless of whether DCs are primed and loaded in culture, or a cutaneous route, DC drain to lymphoid tissue for subsequent T cell activation and expansion. (4) Similar to PBMC isolation for DC preparation, autologous T cells can also be isolated from this pool. These cells can be (re-)stimulated with plate-bound anti-human CD3ε, without anti-CD28, as to only (re-)engage the experience T cells that might respond to TAAs. This CD3ε-targeted stimulation can be further boosted by adding in cytokines that favor a proinflammatory/antitumor T cell subset such as IL-2, IL-12 and IL-15. (5) An alternative to simple autologous T cell isolation, (re-)priming and expansion, is by engineering the T cell to be highly specific to a GBM-expressed antigen, in the form of a chimeric antigen receptor-expressing T cell. This step provides a high level of stringency and targeting for all T cell adoptively transferred into the GBM patient. Ultimately, the goal of all vaccines is to eventually cause GBM-specific T cells to infiltrate the tumor and elicit immune-mediated rejection.
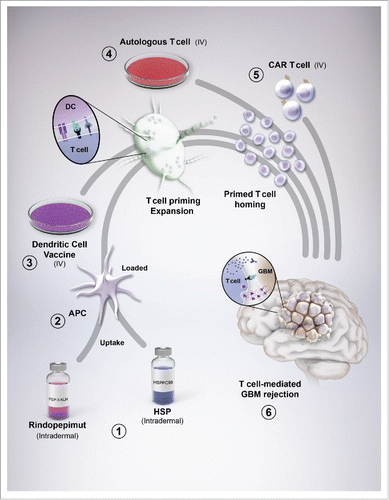
Table 1. Clinical efficacy of vaccines for patients with newly diagnosed adult GBM or pediatric DIPG. *Trial closed ahead of stated objectives.
Table 2. Factors that limit patient selection for vaccine therapy.
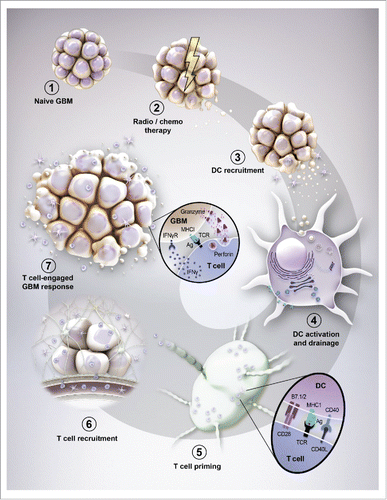
Figure 2. Evolution of brain tumor immunity. (1) The naive (newly diagnosed) brain tumor flourishes in the immunosuppressive environment of the central nervous system and is relatively non-immunogenic. (2) Standard of care for patients with brain tumors causes cancer cell death and the subsequent release of antigens. (3) The inflammation caused by cancer cell death triggers the recruitment of dendritic cells to the brain tumor microenvironment. (4) CD8α dendritic cells (DC) engulf the cancer-associated antigens and utilize the cross-presentation pathway to facilitate the loading of those epitopes onto major histocompatibility complex (MHC) I molecules. The CD8α-loaded DC subsequently emigrate out of the tumor microenvironment while simultaneously increasing costimulatory molecules that facilitate the future productive interaction(s) of naive CD8+ (and CD4+ T cells through MHCII) T cells. (5) After immigration into the lymph node, DC-mediated T cell priming and expansion occurs through MHC/antigen (Ag) expressed by DC and a high affinity T-cell receptor (TCR) expressed by T cells. (6) T cells that are now activated and specific to tumor-associated antigens then emigrate from the lymphoid tissue, back into the circulation. (7) To successfully penetrate the brain tumor, T cells must first come into contact with selectins and integrins (not shown) that facilitate the attachment to inflamed endothelium. Upon successful adhesion, T cells extravasate through the endothelial basement membrane, followed by the perivascular space and eventually through the parenchymal basement membrane whereby they can now come into direct contact with the CNS-resident tumor cells. (8) Productive T cell-mediated GBM rejection occurs when lymphocytes are reactivated by (re-)stimulation of T cell-expressed TCR with MHC/Ag expressed by brain tumor cells. This interaction facilitates the robust production and release of proinflammatory, interferon-gamma (IFNγ), in addition to the release of the pore forming complex, perforin, which facilitates the passive transfection of granzyme molecules (serine proteases) that cleave tumor-expressing procaspases into active molecules that trigger apoptosis.