Figures & data
Figure 1. Co-stimulation with PD-L1-derived epitopes enhances the frequency of T cells reactive against dendritic cell vaccine. (A) PBMCs (5 × 106) from patients were stimulated twice in vitro with DCvacc. The following day, the cultures were co-stimulated with one or two long PD-L1 epitopes or incubated with an irrelevant HIV control peptide. All cultures were stimulated with IL2 the day after peptide stimulation. Cultures were examined for DCvacc-reactive T cells after 16–20 d by intracellular TNFα/INFγ staining. (B–E) Examples of PBMC cultures from three melanoma patients in which co-activation of PD-L1 specific T cells significantly boosted T cell immunity toward DCvacc, as measured by intracellular TNFα/INFγ staining. (B) CD4+ T cells released only TNFα in response to DCvacc. (C) PD-L1 peptide co-stimulation induced TNFα/INFγ double-positive CD4+ T cells in response to DCvacc. (D, E) Co-stimulation with PD-L1 epitopes increased the number of both CD4+ and CD8+ cells that reacted against DCvacc.
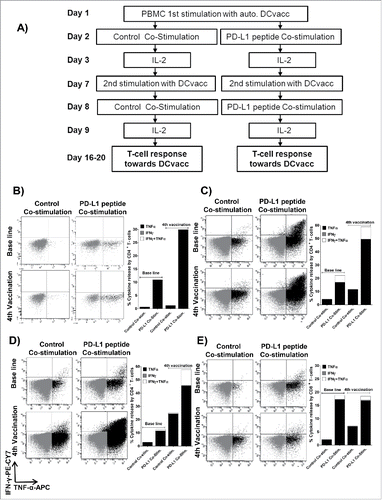
Figure 2. Co-stimulation of PBMCs with PDLong1 plus dendritic cell vaccine. At days 16–20, after two stimulations with DCvacc and two stimulations with either an irrelevant control peptide or PDLong1 peptide, the percentage of cells that released TNFα/INFγ in response to DCvacc was identified by flow cytometry. Percentages of DCvacc-reactive CD4+ T cells (A) and CD8+ T cells (B) in cultures of PBMCs taken from eight melanoma patients before vaccination (baseline) and after four vaccinations.
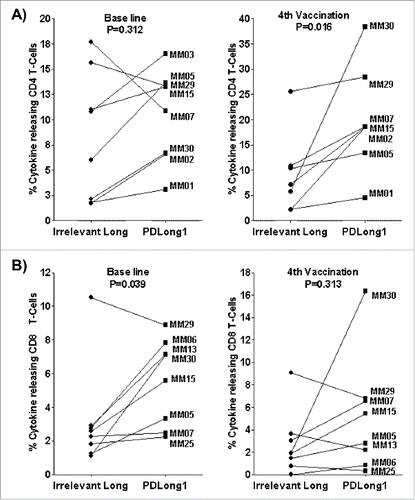
Figure 3. Natural T cell responses to PDLong1 and PDLong2. (A) IFNγ ELISPOT was used to measure T cell response toward PDLong1 and PDLong2 in tumor-infiltrating lymphocytes from melanoma patients. In 5 × 104 cells from 12 melanoma patients, the average number of IFNα-releasing cells in response to either PDLong1 or PDLong2 was measured. (B) Example of ELISPOT wells performed with TILs from two melanoma patients either without or with PDLong1 or PDLong2 peptides (C) Tumor-infiltrating lymphocytes were cultured for 5 h either without or with PDLong1 or PDLong2 before being analyzed for intracellular IFNγ/TNFα staining. (D) Example of IFNγ/TNFα staining of tumor-infiltrating lymphocytes from a melanoma patient in response to either without peptide or with PDLong1 or PDLong2.
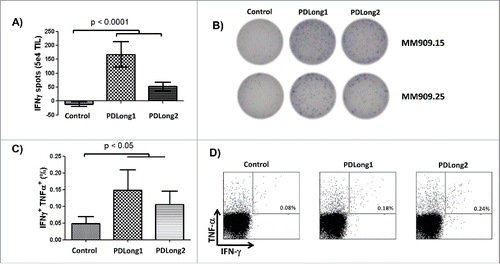
Figure 4. Stimulation of patient PBMCs with PDLong1 plus PDLong2 together with a DC vaccine. At days 16–20, after two stimulations with DCvacc and two stimulations with either an irrelevant control peptide or PDLong1 plus PDLong2 peptide, the percentage of cells that released TNFα/INFγ in response to DCvacc was identified by flow cytometry. Percentages of DCvacc-reactive CD4+ T cells (A) and CD8+ T cells (B) in cultures of PBMCs taken from eight melanoma patients before vaccination (baseline) and after four vaccinations.
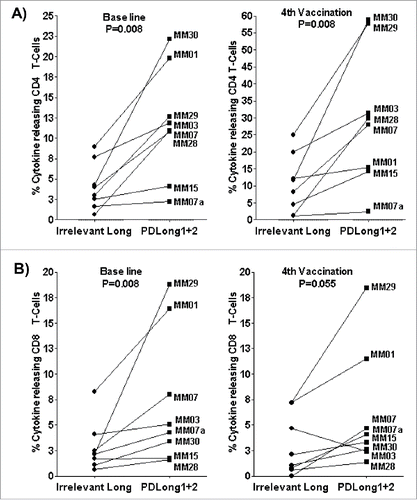
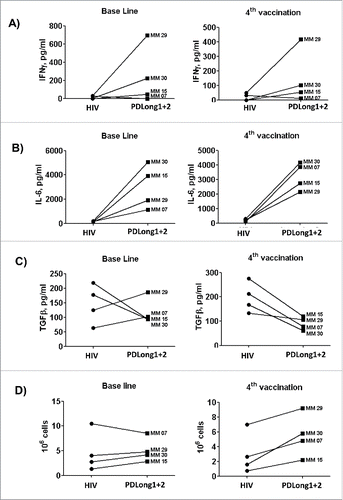
Figure 5. Comparison of cytokine secretion in supernatants from cultures of cells from four patients. Supernatants from cultures either co-stimulated with an irrelevant control peptide or with PDLong1 plus PDLong2 were collected on the day of analysis of DCvacc-reactive T cells so that the presence of INFγ (A), IL-6 (B), or TFGβ (C) could be measured. (D) In addition, the total numbers of cells were counted after the second stimulation with either HIV or PDLong1 plus PDLong2 epitopes.