Figures & data
Figure 1. The combination of IL-21 supplementation at start of cultivation, IL-2 and irradiated EBV-LCL feeder cells enabled a long-lasting, pronounced expansion of NK cells. (A) NK cells were cultivated with IL-2 (black bars) or with IL-2 and 100 ng/mL IL-21 (gray bars) in the presence or absence of irradiated EBV-LCL feeder cells. The expansion of NK cells was measured after 7 d. Displayed are mean values and standard deviation of eight NK cell donors and statistical significance was tested by paired Student's t-test. (B) NK cells were labeled with CellTrace Violet Proliferation Dye and cultivated with IL-2 (dark gray histograms) or IL-2 and 100 ng/mL IL-21 (light gray histograms) in the presence or absence of irradiated EBV-LCL feeder cells. The dilution of CellTrace Violet corresponding to cell proliferation was analyzed by flow cytometry at day 3 and day 5. A representative donor out of three donors is shown. (C) NK cells were co-cultured with irradiated EBV-LCL feeder cells and IL-2 at different concentrations of IL-21. Mean NK cell fold expansion and standard deviation are displayed for three donors. (D) NK cells were co-cultured with irradiated EBV-LCL feeder cells and IL-2. IL-21 (100 ng/mL) was supplemented only at day 0 (gray dots) or permanently (gray squares), meaning at day 0, 7, 9, 11, 13, 16, 18, 20, and 26 when fresh medium was added. Mean NK cell expansion fold and standard deviation of six donors are shown at different time points. (E) NK cells were cultured with IL-2 and 100 ng/mL IL-21 was added at day 0. NK cells were co-cultured with irradiated EBV-LCL and re-stimulated with EBV-LCL at day 13, 26, and 39 (gray line and dots). In addition, the expansion without EBV-LCL restimulation at day 13, 26, or 29 was determined and monitored for 7 d (gray dashed line and triangles). Shown are mean and range of the NK cell expansion folds of six donors at different time points.
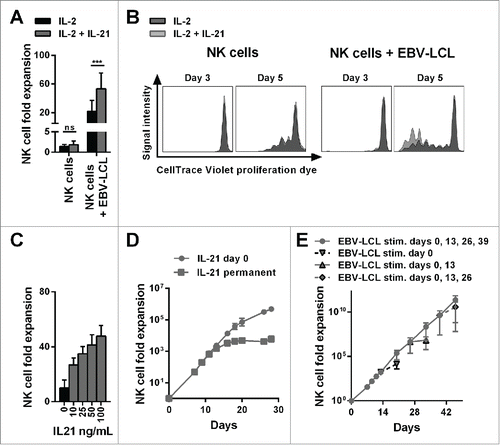
Figure 2. Ex vivo-expanded NK cells were highly cytotoxic against different tumor cell lines and showed enhanced degranulation and production of IFNγ and TNF-α. (A) Different NK cells were tested for cytotoxicity against four tumor cell lines using a standard 51Chromium release assay. Specific lysis at different effector-to-target (E:T) ratios is shown for freshly isolated NK cells (black triangles and dashed line) and NK cells that have been expanded for 13 or 14 d, either with IL-2 (back line and dots) or by use of IL-2, irradiated EBV-LCL and IL-21 supplemented at day 0 (gray line and diamonds). Displayed are mean values and standard deviation of NK cells from 4–8 donors per target cell line and statistical significance was tested by Student's t-test. (B) NK cells were tested for degranulation and production of IFNγ and TNF-α by flow cytometry upon stimulation with PMA/Iono. Shown are data for freshly isolated, naive NK cells (gray and white dotted bars) and NK cells that have been expanded for 13 or 14 d, either with IL-2 (black bars) or by use of IL-2, irradiated EBV-LCL and IL-21 supplementation at day 0 (gray bars). Displayed are mean values and standard deviation of NK cells from five donors. Statistical significance was tested by paired Student's t-test. (C) NK cells were expanded ex vivo for 14 d by use of IL-2, irradiated EBV-LCL and IL-21 supplementation at day 0. Then, NK cells were stimulated for 24 h with PMA/Iono or by co-culture with SK-MEL-28 cells. Supernatants of the cell cultures were harvested and analyzed for the concentrations of 12 different cytokines using a multiplex bead-array assay. Mean and standard deviation for NK cells from three different donors are displayed. Statistical significance was tested by paired Student's t-test.
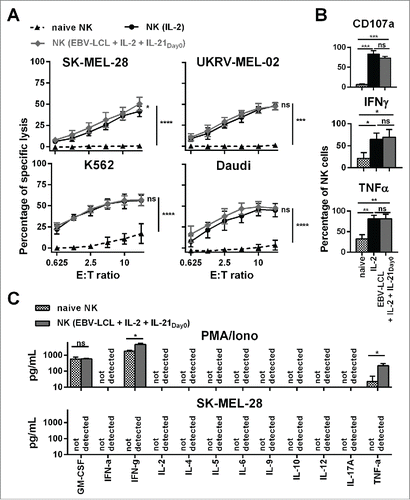
Figure 3. Adoptive transfer of NK cells expanded by the optimized protocol in tumor-bearing mice resulted in pronounced tumor growth control. (A) Scheme is shown for evaluation of expanded NK cells in vivo using a xenograft model. Mice were irradiated and received human SK-MEL-28 melanoma cells-expressing luciferase by intravenous (i.v.) injection. Three days later, after tumor engraftment, the mice were treated (i.v.) with human NK cells expanded with the optimized protocol and the tumor load was monitored by luciferase activity. IL-2 was repeatedly injected intraperitoneally. (B) Tumor-bearing mice, as described in A, were treated with PBS, as a control, or with 30 × 106 NK cells that were expanded for 14 d using the optimized expansion protocol. The pictures display the bioluminescence (radiance) showing the in vivo luciferase activity of four representative mice of each group at the day of NK cell transfer (top) and 13 d thereafter (bottom). (C) Tumor-bearing mice were treated with NK cells as described in B using different NK cell doses. Mean and range of the tumor burden, measured by bioluminescence, is shown at different time points for one representative experiment with 4–5 mice per group. (D) Mice were treated as described in C, and the transferred human NK cells were re-isolated from blood and lungs of the mice 14 d after NK cell injection and were enumerated using flow cytometry. Mean and standard deviation of NK cell numbers per lung and per mL of blood are shown for four mice per group. (E) Tumor-bearing mice were treated with 30 × 106 expanded NK cells as described in B and at day 3, 7, and 14 the mice were sacrificed and human NK cells were re-isolated from blood and lungs. Mean and standard deviation of NK cell numbers per lung or mL of blood are shown for four mice per group. Statistical significance in all experiments was tested by Student's t-test.
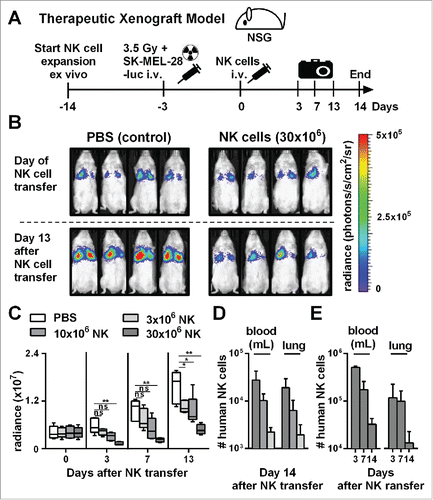
Figure 4. Already three days after transfer into NSG mice, ex vivo-expanded NK cells changed their phenotype and lost their cytotoxicity and potential for degranulation, while they retained an enhanced ability to produce IFNγ and TNF-α. (A) Scheme for characterization of three types of NK cells from the same donor. First, freshly isolated, naive NK cells were analyzed (black). Second, NK cells were expanded ex vivo for 17 d by use of short-term IL-21 stimulation, IL-2 and irradiated EBV-LCL feeder cells (red). Third, NK cells were expanded in the same way for 14 d before they were transferred to tumor-bearing mice (30 × 106 NK cells per mouse) (blue). Three days after the transfer, the transferred human NK cells were recovered from the mouse lungs and NK cells from 3–4 mice were pooled per donor (blue). (B) The differentially prepared NK cells, as described in A, were analyzed for the surface markers NKG2D, TRAIL, and DNAM-1 by flow cytometry. Histograms for one representative NK cell donor are shown (back) together with isotype controls (gray). (C) The flow cytometric analysis, as described in B, is applied for three different donors. For each marker the mean and standard deviation of all donors are shown for the mean fluorescence intensity (MFI) corrected by isotype subtraction (top) and the frequency of NK cells expressing the marker (bottom). (D) The differentially prepared NK cells, as described in A, were analyzed for cytotoxicity against K562 and SK-MEL-28 target cell lines at a 3:1 effector-to-target ratio in triplicates per donor. One out of two representative experiments is shown using two different NK cell donors. (E) The differentially prepared NK cells, as described in A, were analyzed for degranulation and production of IFNγ and TNF-α upon stimulation with PMA/Iono. Mean and standard deviation of three different NK cell donors are displayed. Statistical significance was tested by paired Student's t-test in all experiments.
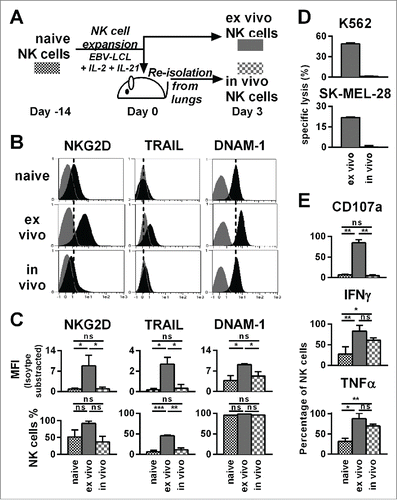
Figure 5. The presence of tumor does not influence the phenotype, potential for degranulation, and cytokine production of adoptively transferred NK cells. (A) A scheme is shown for evaluation of expanded NK cells in vivo. Mice were irradiated and received human SK-MEL-28 melanoma cells (with tumor) or PBS (w/o tumor) by intravenous (i.v.) injection. Three days later after establishment of tumor, all mice were treated (i.v.) with human NK cells expanded with the optimized protocol. Three days after transfer of the NK cells, cells from the blood and lungs of the mice were recovered. (B) NK cells from the blood and lungs were enumerated and NK cell numbers per g of lung and per mL of blood are shown. (C) NK cells from mouse lungs as described in A were analyzed for the surface markers NKG2D, TRAIL, and DNAM-1 by flow cytometry and shown are the mean fluorescence intensity (MFI) corrected by isotype subtraction. (D) NK cells as described in A were stimulated with PMA/Iono and analyzed by flow cytometry for degranulation and production of IFNγ and TNF-α. Means and standard deviations for NK cells from three independent donors are shown with cells from 3–5 mice that were pooled per condition for each donor. Statistical significance was tested by paired Student's t-test.
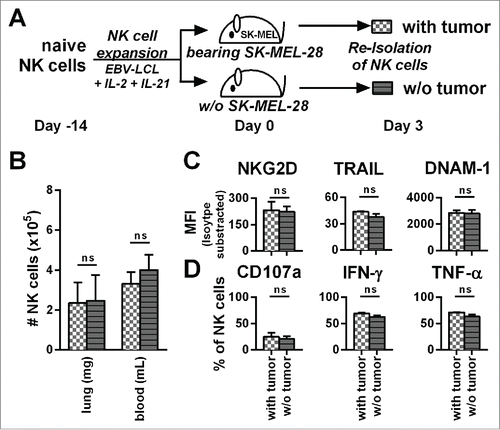
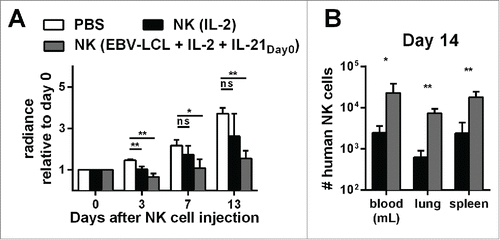
Figure 6. Expansion of NK cells using the optimized protocol (IL-21 day0, IL-2 and irradiated EBV-LCL) results in NK cells with better in vivo persistence and antitumor activity compared to IL-2-expanded NK cells. (A) Mice-bearing SK-MEL-28 cells expressing luciferase either received intravenous (i.v.) PBS (white bars) or human NK cells. The injected NK cells were previously expanded ex vivo for 14 d, either with IL-2 (black bars) or with IL-2, irradiated EBV-LCL and IL-21 supplemented at day 0 (gray bars). In total, three independent NK cell donors were used in different experiments and the bioluminescence (radiance) of each mouse at day 3, 7, and 13 was measured and analyzed relative to the bioluminescence at day 0. For each donor, 2–8 mice were used per group, the mean and standard deviation of all donors are shown at different time points. (B) Tumor-bearing mice were treated as described in A and human NK cells were re-isolated from blood, lungs, and spleen of the mice 14 d after NK cell injection. NK cells were enumerated using flow cytometry and mean and standard deviation of NK cell numbers are shown for one representative donor using four mice per group. Statistical significance was tested by Student's t-test in all experiments.