Figures & data
Figure 1. An immunosuppressive tumor and/or lymph node environment could be prognostic and impact responses to therapies in luminal B breast cancer. The figure depicts a tumor juxtaposed against a draining lymph node. The tumor milieu contains malignant cells which likely express checkpoint molecules such as PD-L1, and secrete suppressive molecules such as TGFβ and VEGF that limit T cell function and promote angiogenesis. Tumor-associated macrophages may initially represent pro-inflammatory, antitumor M1 macrophages (M1) that secrete TNF, IFNγ and other pro-inflammatory cytokines. However, in response to tumor-derived factors, M1 macrophages or newly recruited macrophages may polarize to anti-inflammatory, pro-tumor M2 macrophages (M2), or to an intermediate M3 macrophage (M3), both of which could express PD-L1 +/− Gal-9 or other checkpoint molecules, and secrete suppressive molecules such as TGFβ and IL-10, which together prevent effector CD8+ T cell function, particularly T cells expressing checkpoint molecules such as PD-1 and TIM-3. Myeloid-derived suppressor cells (MDSCs) augment the suppressive milieu by expressing and secreting similar immune suppressive molecules. Tumor-infiltrating dendritic cells (DCs) may take up whole tumor cells or tumor cell debris and traffic to lymph nodes. If, in response to the tumor microenvironment, DCs express checkpoint molecules such as PD-L1 and/or Gal-9, their ability to activate tumor-specific effector T cells is impaired, and they may instead induce Tregs. The presence of M2 macrophages in tumor-draining draining lymph nodes may further induce dysfunctional T cells expressing checkpoint inhibitors or Tregs.
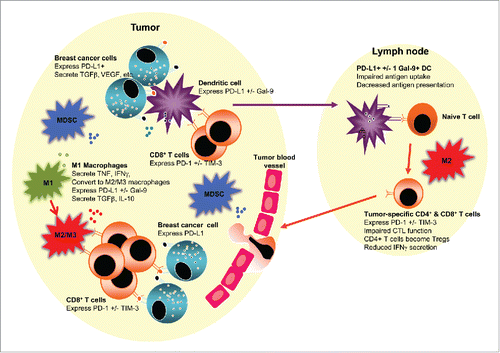
Table 1. Checkpoint molecules and their ligands.
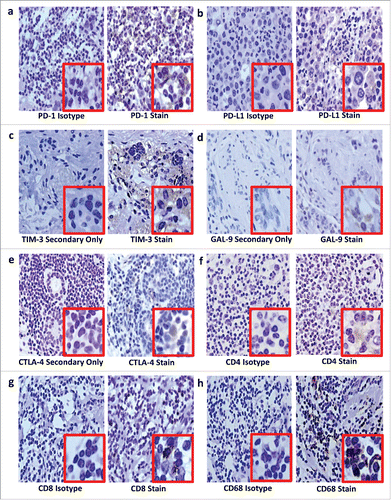
Figure 2. Immunohistochemical staining on luminal B breast cancer tissue for PD-1 (a), PD-L1 (b), TIM-3 (c), GAL-9 (d), CTLA-4 (e), CD4+ (f), CD8+ (g) and CD68 (h). After antigen retrieval on deparaffinized rehydrated sections, endogenous peroxidases were blocked using hydrogen peroxide (Riedel-de Haën, Seelze, Germany) while endogenous avidin and biotin were blocked using an avidin/biotin blocking kit (Invitrogen, Camarillo, USA). Sections were sequentially incubated with a protein block containing fetal calf serum, bovine serum albumin and human plasma to prevent background staining; then incubated with unconjugated primary antibodies or isotype controls; washed; linked first to biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, USA) or biotinylated goat anti-mouse IgG1 (Biolegend, San Diego, USA) secondary antibodies, then to streptavidin-conjugated horseradish peroxidase. Staining was visualized using hydrogen peroxide in 3,3-diaminobenzidine (Sigma Aldrich, St Louis, USA) counterstained with Harris' haematoxylin. Sections were dehydrated before mounting with Entellan mounting media (Merck Millipore, Darmstadt, Germany). In place of isotype controls for the polyclonal rabbit primary antibodies, TIM-3 (c), GAL-9 (d) and CTLA-4 (e), a secondary antibody-only stain was prepared. Curtin University (Perth Western Australia) and Bellberry (South Australia) Human Ethics Committees approved this study (approval numbers HR 107/2015 and 2015–03–151, respectively).