Figures & data
Figure 1. PyMT/Il1r1−/− mice have an earlier tumor onset and increased metastasis compared with PyMT mice. (A) Kaplan–Meier tumor-free survival curves of PyMT (median = 99 d) or PyMT/Il1r1−/− mice (median = 72.5) (n = 10–20 per genotype, Mantel–Cox p < 0.0001). (B) Kaplan–Meier survival curves of PyMT (median = 170 d) or PyMT/Il1r1−/− mice (median = 140.5) (n = 20–30 per genotype, Mantel–Cox p < 0.0001). (C) Number of mammary gland with tumors at clinical end point (n = 15–20, Student's t-test p < 0.001). (D–F) Primary tumor burden (D), percentage of mice bearing metastasis (E) and average number of lung lesions per metastasis-bearing animals (F) at 5 mo of age of PyMT or PyMT/Il1r1−/− mice (n = 7–19 mice per genotype). Each symbol represents a mouse; the line represents the mean. (G-I) Primary tumor burden (G), percentage of mice bearing metastasis (H) and average number of lung lesions per metastasis-bearing animals (I) at clinical end point of PyMT or PyMT/Il1r1−/− mice (n = 16–17 mice per genotype, Student's t-test p < 0.01). Each symbol represents a mouse; the line represents the mean.
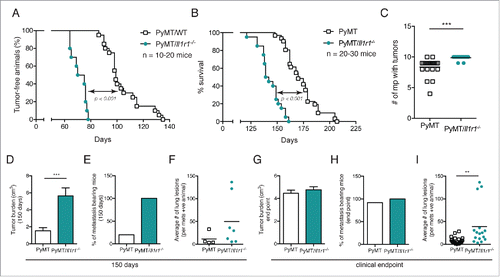
Figure 2. Increased foci coverage in PyMT/Il1r1−/− mice compared with PyMT mice at early time points. (A) Whole mounts of mammary fat pads of PyMT or PyMT/Il1r1−/− mice at 6 and 10 weeks of age were stained with hematoxylin and analyzed for foci coverage (n = 4–5 mice per time point per genotype, Student's t-test p < 0.001). (B) Representative pictures of whole mount of PyMT or PyMT/Il1r1−/− mice at 6 and 10 weeks of age. Boxed region represents insets. (C) H&E of mammary fat pad section of PyMT or PyMT/Il1r1−/− mice at 10 weeks of age. (D) Western blots depicting E-cadherin, Cyclin D1 and GAPDH expression in lysates from mammary fat pads of WT, Il1r1−/−, PyMT or PyMT/Il1r1−/− mice at 6 weeks of age. (E) Densitometry analysis of the ratios of E-cadherin/GAPDH and Cyclin D1/GAPDH, using the ImageJ software. (F) Mice were palpated twice a week to determine the first appearance of tumors. Tumor volume was measured weekly for the following 6 weeks (n = 8–12 mice per genotype, Student's t-test per time point).
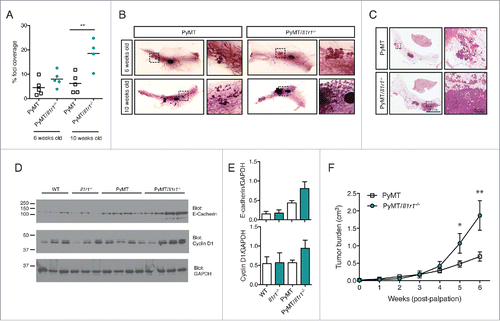
Figure 3. Increase in proliferation rate, but not cell death, in PyMT/Il1r1−/− mice at early time points. (A) PyMT or PyMT/Il1r1−/− mice at 10 weeks of age were injected i.p. with BrdU 2 h before sacrifice. Epifluorescence images of mammary fat pad were stained with antibodies against BrdU and EpCAM. Mammary ducts were classified into four different grades (grade 0 = healthy, grade 1 = hyperplastic ducts, grade 2 = early adenoma, and grade 3 = late adenoma/carcinoma). (B) Quantification of BrdU and EpCAM double positive nuclei, determined using Volocity, and represented as a percentage of total EpCAM positive nuclei. Each symbol represents one duct (n = 3 mice per genotype, Student's t-test). (C) Representative images of PyMT or PyMT/Il1r1−/− mice of a grade 2 mammary duct. (D) Epifluorescence images of mammary fat pads from 10-week-old mice were stained with TUNEL as a measure of cell death. (E) Quantification of TUNEL positive nuclei, determined using Volocity (n = 2–3 mice per genotype, Student's t-test).
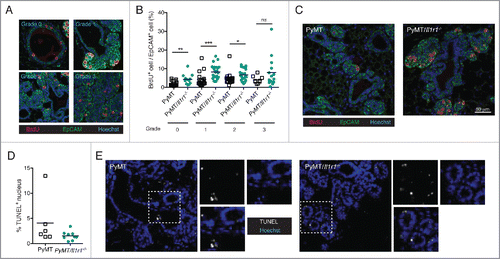
Figure 4. Il-1r1 ablation does not affect the proportions of immune infiltrates in the mammary fat pad during early or late tumorigenesis. (A) Flow cytometric analysis of immune infiltrates as a percent of total CD45+ cells in mammary fat pad of PyMT or PyMT/Il1r1−/− mice at 6 and 10 weeks of age (n = 7–8 mice per genotype, Student's t-test). (B) Flow cytometric analysis of intra-tumoral immune infiltrate populations as a percent of total CD45+ cells in mammary carcinomas of PyMT or PyMT/Il1r1−/− mice when tumors reached a volume of 1 cm3 (n = 5 mice per genotype, Student's t-test).
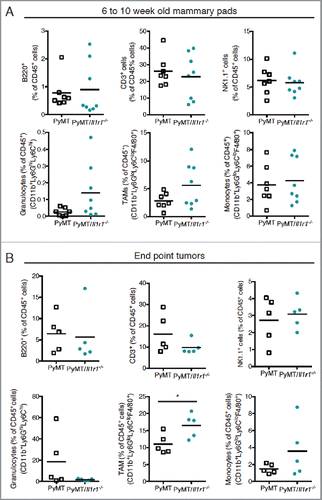
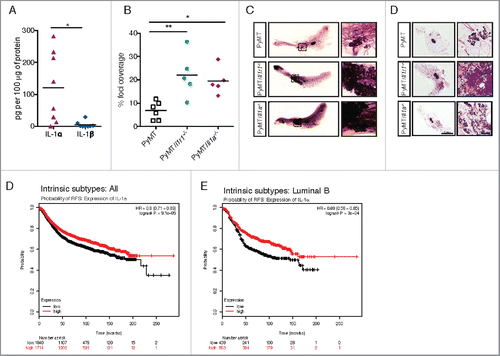
Figure 5. IL-1α expression leads to decreased foci coverage in mice and correlates with better patient survival. (A) ELISA for IL-1α and IL-1β were performed on lysates from ∼1 cm3 mammary tumors from PyMT mice. Each symbol on the graph represents one mouse; the horizontal line represents the mean. (B) Whole mounts of mammary fat pads from PyMT, PyMT/Il1r1−/− or PyMT/Il1a−/− mice at 10 weeks of age were stained with hematoxylin and analyzed for foci coverage (n = 4–5 mice per time point per genotype, Student's t-test p < 0.001). (C) Representative pictures of whole mounts of mammary fat pads from PyMT, PyMT/Il1r1−/− or PyMT/Il1a−/− mice at 10 weeks of age. Boxed region represents insets. (D) H&E of mammary fat pad section from PyMT, PyMT/Il1r1−/− or PyMT/Il1a−/− mice at 10 weeks. Boxed region represents insets. (E, F) Kaplan–Meier survival curves showing probability of relapse-free survival (FRS) in patients with all intrinsic subtypes (E) or luminal B (F) breast cancer with low or high expression of IL-1α expression. The optimal expression threshold was auto-selected by K–M plotter software. Hazard ratio (with confidence intervals) and log-rank probability values are included on each graph.