Figures & data
Figure 1. Early and late administration of VIPhyb improves survival in AML and T cell leukemia-bearing mice. Albino B6 mice were administered 10 μg VIPhyb subcutaneously for 7seven consecutive days beginning either the day before or one week after receiving 106 C1498 or 2 × 106 LBRM cells intravenously. (A) Timeline of tumor injection and VIPhyb treatment. (B) Survival of LBRM-bearing mice receiving either PBS or VIPhyb. (n = 10 PBS, n = 10 VIPhyb) (C) Survival of C1498-bearing mice receiving either PBS or VIPhyb. (n = 16 PBS, n = 14 VIPhyb) (D) Survival of WT and VIP KO mice following C1498 inoculation. (n = 10 WT, n = 10 VIP KO) (E) Survival data from two experiments of C1498-bearing mice receiving late treatment with PBS or VIPhyb. (n = 20 PBS, n = 20 VIPhyb) *p < 0.05 and ***p < 0.001 indicate significant differences between the control and treated groups.
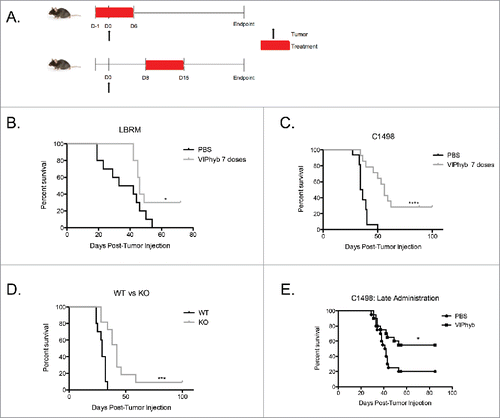
Figure 2. VIPhyb treatment led to reduced tumor burden in mice, which required the presence of lymphocytes. C1498-bearing mice were injected i.p with luciferin, anesthetized, and imaged in an IVIS spectrum imager. Rag1 knockout mice and wild-type albino B6 mice were injected with 106 C1498 cells i.v and treated with an early course of VIPhyb or PBS. (A) Representative BLI image of late stage C1498-bearing albino B6 mice treated with an early course of either PBS or VIPhyb. The scale indicates the intensity of the signal emitted from C1498 cells. (B) Quantification of tumor burden reported as average flux emitted from each mouse's entire body. (C) Survival of C1498-bearing, VIPhyb-treated Rag1 knockout mice compared with wild-type C1498-bearing B6 albino mice treated with either PBS or VIPhyb. (n = 9 PBS, n = 10 VIPhyb, n = 4 Rag1 KO PBS, n = 8 Rag1 KO VIPhyb) The red box on the x-axis indicates the treatment period. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences between or among groups of mice.
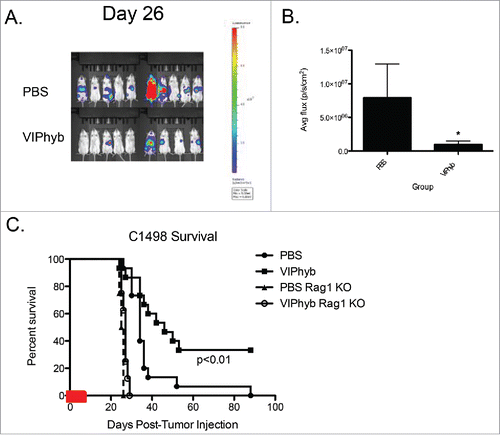
Figure 3. VIPhyb reduced CREB phosphorylation in T cells following VIP stimulation and enhanced proliferation in vitro. Splenic T cells were purified by negative selection and cultured in complete media containing 0.5% fetal bovine serum overnight to reduce basal CREB phosphorylation levels. Cells were then incubated in the presence of 10 μM VIPhyb before stimulation with 1 nM or 100 pM VIP. Levels of CREB phosphorylation were then assessed by Phosflow and Western blot. The effect of VIPhyb on the in vitro proliferation of T cells was assessed by culturing CFSE-labeled splenic T cells in a 96-well plate pre-coated with anti-CD3 antibody. CFSE dilution was measured 48 h later by flow cytometric analysis of live CD3+ cells. (A) Representative histograms indicating levels of CREB phosphorylation in VIP-stimulated CD4+ and CD8+ T cells. The solid gray histogram represents the isotype, the black histogram represents unstimulated samples, the red histogram represents samples stimulated with VIP, and the blue histogram represents samples pre-incubated with VIPhyb before VIP stimulation. (B) Western blot showing CREB phosphorylation in splenic T cells treated with 1 nM VIP or 10 μM VIPhyb + 1 nM VIP. (C) Histogram indicating the level of CFSE dilution in control wells and wells containing 1 nM VIP with or without 10 μM VIPhyb. The solid gray histogram represents T cells cultured in wells without anti-CD3, the black histogram represents control wells not containing peptide, the red histogram represents wells containing VIP, the blue histogram represents wells containing VIPhyb alone, and the green histogram represents wells containing VIP and VIPhyb. (D) Quantification of CFSE dilution normalized to control wells for three independent experiments. *p < 0.05, **p < 0.01 indicate significant differences among culture conditions.
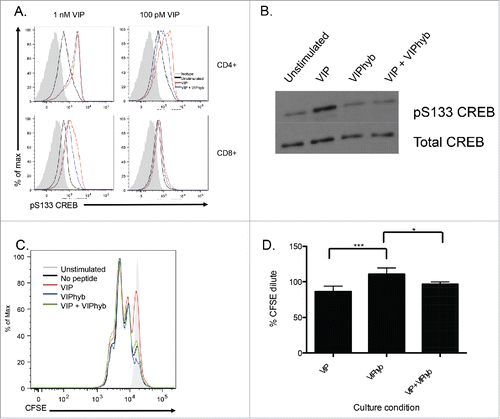
Figure 4. C1498 cells express VPAC2 but are not directly affected by VIPhyb treatment. RNA and protein from cultured C1498 cells were extracted, and expression of VPAC1 and VPAC2 was determined by RT-PCR and Western blot. C1498 cells were cultured in the presence or absence of VIPhyb or daunorubicin as positive control. Survival of cells was measured by annexin V-sytox blue staining and flow cytometry while growth of the cells was measured by BLI. Expression of PD-L1 was determined by flow cytometry following 48-h culture in the presence of 10 ng/mL IFNγ and various concentrations of VIPhyb. (A) Image of PCR products run on a 1% agarose gel with 18s rRNA as control. (B) Image of Western blot probed for VPAC1 and VPAC2. (C) Growth curves of C1498 cells under various culture conditions as measured by BLI. (D) Flow cytometry plots of C1498 cells cultured with 1 μM VIPhyb, 1 μM daunorubicin, 1 nM VIP, or no drug as control. Cells in the lower right quadrant are in early apoptosis while cells in the upper right quadrant are dead. (E) Expression of PD-L1 on live C1498 cells cultured for 48 h in the presence of IFNγ and VIPhyb.
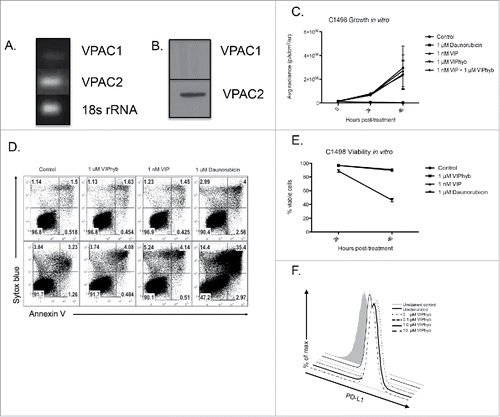
Figure 5. Adoptive transfer of T cells from VIPhyb-treated tumor survivors conferred protection to Rag1 knockout mice. Splenic T cells from VIPhyb-treated mice that survived C1498 were purified by negative selection. 5 × 106 T cells were adoptively transferred to Rag 1 knockout recipients followed by injection of 106 C1498 cells one week later. Peripheral blood samples were analyzed 14 d post-tumor inoculation for the frequencies of T cell subsets. Tumor burden was assessed 21 d post-tumor inoculation by BLI. (A) T cell frequencies in peripheral blood of tumor-bearing Rag 1 KO mice receiving naive T cells or T cells from VIPhyb-treated mice. (B) Frequencies of CD4+ and CD8+ T cells in tumor-bearing Rag 1 KO mice receiving naive T cells or T cells from VIPhyb-treated mice. (C) Flow cytometry plots showing differential expression of the markers CD44 and CD62L on peripheral blood T cells from tumor-bearing Rag 1 KO mice receiving naive T cells or T cells from VIPhyb-treated mice. (D) Quantification of T cell subsets as defined by the expression of CD44 and CD62L. (E) Comparison of tumor burden as assessed by BLI between mice receiving naive T cells and mice receiving T cells from VIPhyb-treated donors. (F) Survival of mice receiving naive T cells vs. mice receiving T cells from VIPhyb-treated donors following a second challenge of 106 tumor cells. (n = 10 naive T cells, n = 6 VIPhyb-treated T cells) *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences between groups.
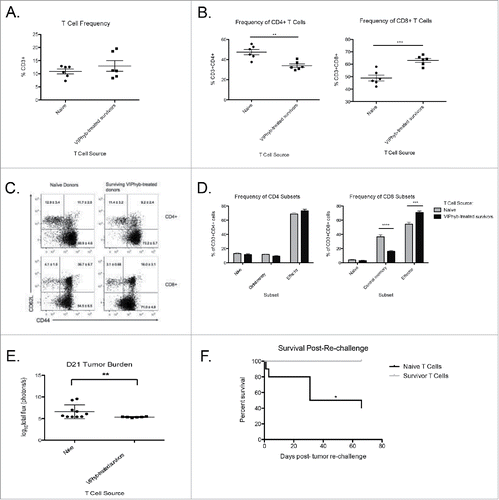
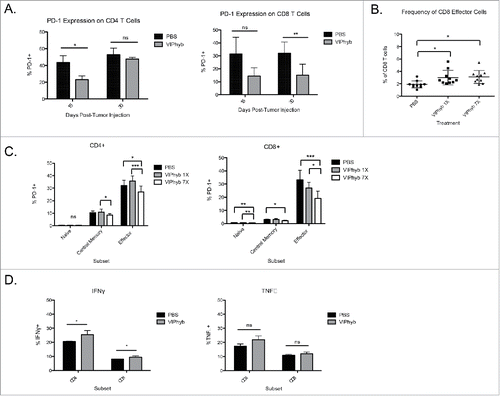
Figure 6. VIPhyb treatment reduced PD-1 expression in CD4+ and CD8+ T cells and enhanced the production of pro-inflammatory cytokines. Peripheral blood and spleen samples were collected on days 15 and 30 post-tumor cell injection and analyzed by flow cytometry. (A) PD-1 expression on total peripheral blood CD4+ and CD8+ T cells at the indicated time points. (B) Frequency of CD44+CD62L− effector CD4+ and CD8+ T cells in peripheral blood 15 d post-tumor cell inoculation. (C) Expression of PD-1 on subsets of peripheral blood CD4+ and CD8+ T cells 15 d post-tumor cell injection as defined by the expression of CD44 and CD62L. Graphs compare the expression of PD-1 in mice treated with PBS, one single dose of VIPhyb, or seven consecutive daily doses of VIPhyb. (D) Expression of IFNγ and TNF-α in splenic T cells from C1498-bearing mice treated with PBS or seven doses of VIPhyb. *p < 0.05, *p < 0.01, and ***p < 0.001 indicate significant differences between groups.