Figures & data
Table 1. Characterization of lung cancer patients and controls.
Figure 1. Western blotting analysis. (1A and 1B): Reactivity of serial serum samples from two representative LC patients in group 1 to LC cell line H1299. Patient 1 was a 69-y-old Caucasian male who was a current heavy smoker (more than 50 pack years) and started to smoke at the age of 16-y-old. He was drawn sequential serum samples between year of 2003 and 2011 and diagnosed as lung adenocarcinoma of stage IA in Oct. 2009. As shown in 1A, in the initial serum sample collected in May 2003, autoantibodies were barely observed, but appeared one year later, 5 y before the diagnosis of LC. The 34 kD band (arrow) arose at the time point of 1.5 y before the lung cancer diagnosis (lane 5 in 1A) and reached at the highest titer at the time of 3 mo before the diagnosis (lane 6 in 1A). Shows a 64-y-old Hispanic male, who has been smoking for 44 y with average 32 pack years, since he was 20 y old and diagnosed as small cell LC in stage IA when he was 64-y-old. A 34 kD reactive band (arrow) was observed in the initial serum sample collected in Sept. 2003 (lane 1 in 1B), but the titer was sharply increased 4 y later that was the time point of 3 y (lane 4 in 1B) before LC diagnosis. (1C) Reactivity of serum samples from five lung cancer patients in group 1, which drawn at the diagnosis of LC and five normal individuals in group 2 to H1299 cell line. 1D-1I: Immunoreaction of recombination protein ECH1 (1D–1F) and HNRNPA2B1 (1G–1I) to the serial serum samples from two representative patients the same with 1A and 1B, and to 10 representative individual sera (1F and 1I) from LC patients in group 3. *Time of lung cancer diagnosis (patient 1 was diagnosed in Oct. 2009 and patient 2 in April 2001).
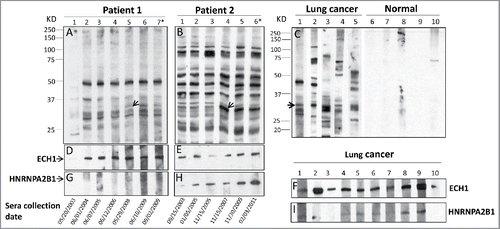
Figure 2. Immunoreactive proteins were detected by autoantibodies in serum samples from lung cancer patients. 2-DE gel electrophoresis of H1299 subcellular fractions (cytosolic, membrane and nucleus) were stained by Coomassie blue (2A, 2D and 2G). 2-DE Western blotting of H1299 subcellular fractions with the pool of sera from LC patients (n = 5 from group 1: line 1–5 in ) (2B, 2E and 2H) and with the pool of sera form normal individuals (n = 5 from group 2: line 6–10 in ) (2C, 2F and 2I).
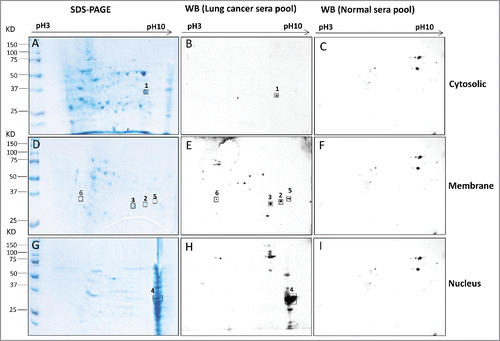
Table 2. Summary of identified protein spots by Mass Spectrometry.
Table 3. Comparison of individual markers in the validation set 2.
Figure 3. Autoantibody levels and receiver operating characteristic (ROC) curves of autoantibody to ECH1, GAPDH and HNRNPA2B1 in validation set 2. 3A–3C: Box and Whisker plots for serum levels of autoantibody to ECH1 (3A), GAPDH (3B) and HNRNPA2B1 (3C) in LC, normal control and COPD patients. The line within the box marks the median, and the 25th and 75th percentiles are presented by the edges of the area, which is known as inter-quartile range (IQR). The bars indicate 1.5 times of the IQR from upper or lower percentiles. 3D–3I: ROC curves of autoantibody to ECH1 (3D–3F) and HNRNPA2B1 (3G–3I). AUC: area under the curve. COPD: chronic obstructive pulmonary disease.
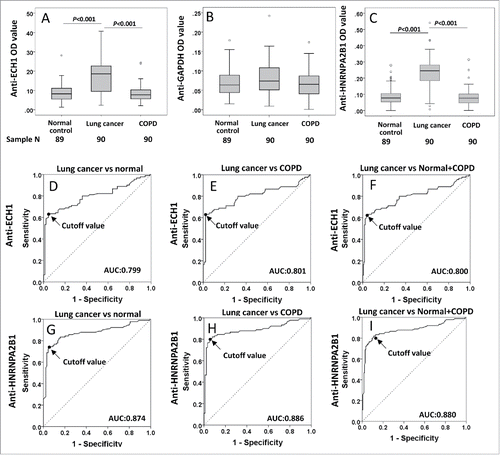
Table 4. Evaluation of autoantibodies to ECH1 and HNRNPA2B1 in lung cancer diagnosis.
Table 5. Autoantibodies against ECH1 and HNRNPA2B1 on effect of patients and disease characteristics in validation set 2.
Figure 4. Correlation of autoantibodies to ECH1 and HNRNPA2B1 with tumor size and lymph node number in lung cancer patients in validation set 2 group 3. 4A–4D: Box and Whisker plots for serum levels of anti-ECH1 in different tumor size (4A) and lymph node number (4C), and anti-HNRNPA2B1 levels in different tumor size (4B) and lymph node number (4D). The line within the box marks the median, and the 25th and 75th percentiles are presented by the edges of the area, which is known as inter-quartile (IQR). The bars indicate 1.5 times of the IQR from upper or lower percentiles. Mann–Whitney test was used to compare the difference of antibody levels in different tumor size and lymph node numbers. 4E–4H: Scatter graph for serum levels of anti-ECH1 in tumor size (4E) and lymph node number (4G), and anti-HNRNPA2B1 levels in tumor size (4F) and lymph node number (4H). Spearman's rank-order test was used to evaluate the correlation of autoantibody level with tumor size and lymph node numbers. LM: lymph node metastasis number.
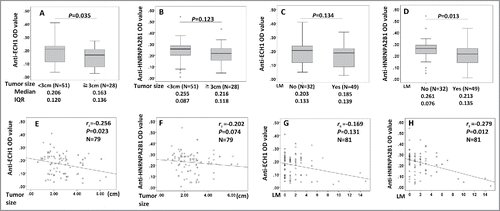
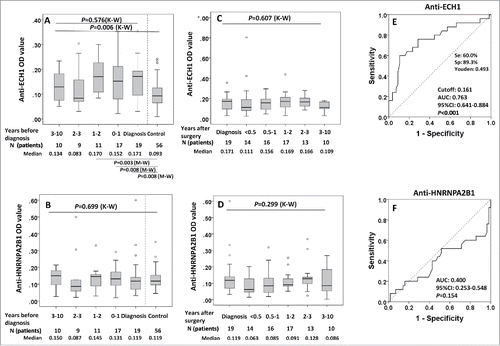
Figure 5. Serial serum analysis of anti-ECH1 and anti-HNRNPA2B1 levels in discovery set 1 study groups. 5A and 5B: Longitudinal analysis of serum anti-ECH1 and anti-HNRNPA2B1 in LC patients at diagnosis and before diagnosis follow-up. Data are presented in quantile box plots. 5C and 5D: Longitudinal analysis of serum anti-ECH1 and anti-HNRNPA2B1 in LC patients at diagnosis, after diagnosis and surgery treatment follow-up. When there was more than one sample in a calendar year in a LC patient, we plotted the average OD for that year. 5E and 5 F: ROC curve of LC patients (n = 28) versus controls (n = 56) for autoantibody to ECH1 (5E) and HNRNPA2B1 (5F). For the patients with serial serum samples before diagnosis, the OD value form the serum samples with collection date closest to diagnosis was used for generating ROC. K–W: Kruscal–Wallis test for multiple groups; M–W: Mann–Whitney test for two groups.