Figures & data
Figure 1. Severe, but not mild heat shock treatment of human cancer cells induces cell death. (A, B) The viability of untreated A549 and OV90 cells and cells treated with mHS (42°C, 1 h) or sHS (47°C, 1 h) followed by an incubation at 37°C for 0 h, 3 h, 6 h and 24 h. (C) Colony-forming assays were assessed after 10 d.
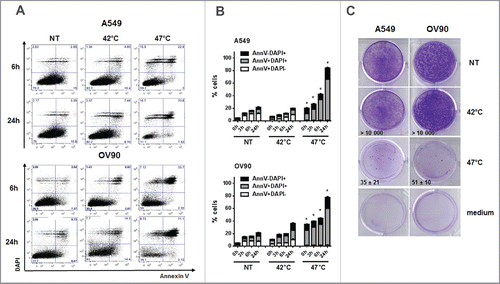
Figure 2. sHS-treated tumor cells expose calreticulin, HSP70 and HSP90, decrease CD47 on the cell surface and release HMGB1 and ATP. A549 or OV90 cells were left untreated or were treated with mHS (42°C, 1 h) or sHS (47°C, 1 h) followed by an incubation at 37°C for 6 h and 24 h. The exposure of HSP70, HSP90, calreticulin and the decrease of CD47 on the cell surface was determined by flow cytometry from AnnV+DAPI− cells (A). (B) Immunoblot of calreticulin cell surface exposure 2.5 h after HT treatment. The expression of PERK, CEA and Her2/neu are shown as controls of cellular and membrane fraction purity. (C) Intra- and extra-cellular levels of ATP 1 h after HS treatment (D) HMGB1 release 24 h after HS treatment. Graphs represent means ± SEM of n = 3–5 (* p < 0.05). Pictures and immunoblots are representative of n = 3–4. Comparison of cell death (E) and calreticulin exposure (F) in A549 and OV90 cells induced by treatment with sHS, UV, idarubicin and cisplatin after 6 h and 24 h. Calreticulin exposure was analyzed by flow cytometry from early (AnnV+DAPI−) and late (AnnV+DAPI+) apoptotic cells (F). Graphs represent means ± SEM of n = 3 (*p < 0.05).
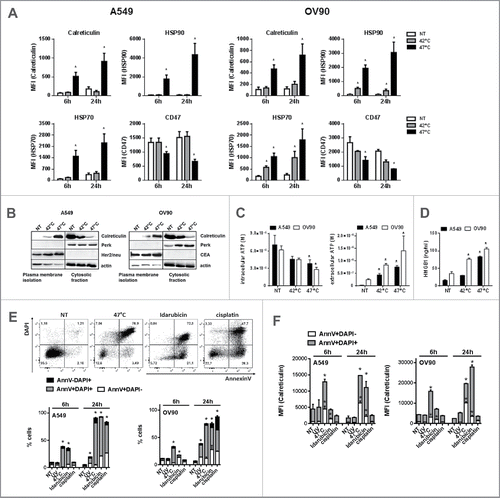
Figure 3. sHS treatment of human cancer cells induces intracellular Ca2+ release, ROS generation and ER stress response leading to a cell death with apoptotic features. HS-induced (A, B) ROS generation after 3 h, (C) time course analyses of ER stress response, (D) intracellular Ca2+ release and (E) the loss of mitochondrial potential after HS treatment (1 h = 0 h) were analyzed. (F) Survival analyses of sHS-treated MEF wt and MEF Bax/Bak −/− cells. (G) Time course analyses of cleavage of caspases, lamin, fodrin and cytochrome c release. (H) Flow cytometry analyses of sHS-treated A549 incubated with capase-9 inhibitor. Dotplots and immunoblots are representative of n = 3–6. Graphs represent means ± SEM of n = 3–5 (*p < 0.05).
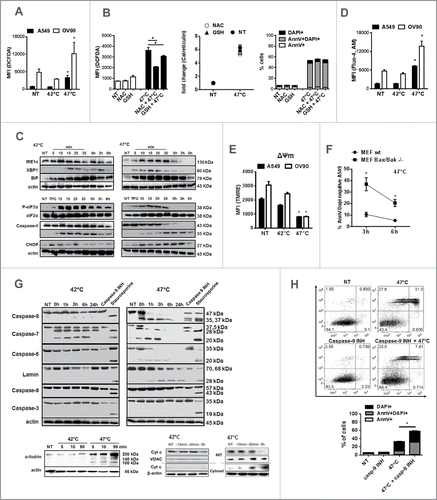
Figure 4. Phagocytosis of sHS-treated tumor cells partially depends on calreticulin and leads to a phenotypic maturation of DC in vitro. (A, B) Untreated cells or cells treated with mHS (42°C, 1 h) or sHS (47°C, 1 h) followed by incubation at 37°C for 24 h were incubated with DC for additional 24 h. Graphs show percentage of DAPI negative DiO+DiD+ DCs and represent means ± SEM of five donors or four mice (*p < 0.05). (C) The expression of maturation markers on DCs after 24 h in cultures where DC + HS-treated tumor cells were co-incubated or where HS-treated cells were separated from DCs by a transwell. (D) IL-10, IL-12p70 and TNF-α production was determined from cell culture supernatants by ELISA after 24 h. The graphs are means ± SEM of 6–8 donors (*p < 0.05).
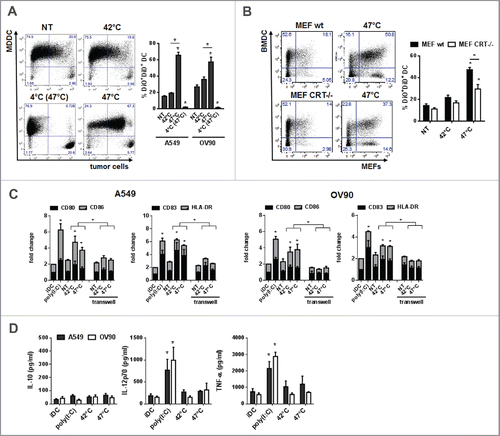
Figure 5. DCs pulsed with sHS-treated tumor cells stimulated antigen-specific CD4+ T cells and induced higher CD8+ T cell activation and proliferation. DCs incubated with sHS or mHS-treated cells for 24 h were added to autologous T lymphocytes at ratio 1:10. The number of IFNγ producing (A) CD8+ and (D) CD4+ T cells, proliferation (Ki67) and activation (CD137) of (C) CD8+ and (D) CD4+ T cells was determined after 7 d after one round of restimulation. (B) The number of MP158–66-specific CD8+ T cells was determined after 9 d. (E) Percentage of Foxp3+CD127low T regulatory cells from CD4+CD25+ T cells. Dotplots are representative of 4–8 donors. Graph shows percentage of IFNγ+CD8+ T cells or IFNγ+CD4+ T cells from CD8+ or CD4+ T cells, respectively and represents means ± SEM of 4–8 donors (*p < 0.05). (F) Immunoblot of tumor antigens after HS treatment. Immunoblots are representative of n = 3.
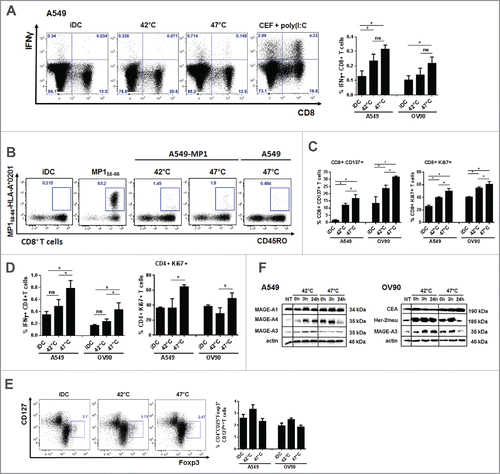
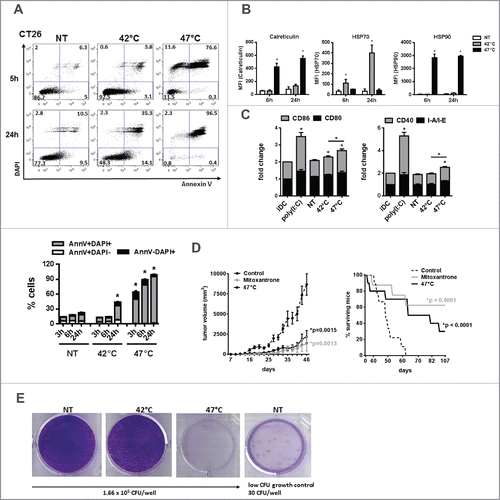
Figure 6. sHS-treated tumor cells are immunogenic and elicit prophylactic immunity in mice. (A, B) The viability of untreated CT26 cells and cells treated with mHS (42°C, 1 h) or sHS (47°C, 1 h) followed by an incubation at 37°C for 5 h and 24 h. (C) The exposure of HSP70, HSP90 and calreticulin was determined by flow cytometry from AnnexinV+DAPI− cells. Graphs represent means ± SEM of n = 3 (*p < 0.05). Dotplots are representative of n = 3. (D) Mice were vaccinated with sHS or mitoxantrone-treated CT26 cells at days 0 and 21 and challenged with live CT26 at day 31. Tumor growth and survival was monitored in 10 mice per group. Three out of 10 mice were considered long-term survivors after day 100. The results are representative of n = 3. (E) Colony-forming assays were assessed after 14 d.