Figures & data
Figure 1. IL-21R gene expression and transcript levels are upregulated on NK cells following FcR stimulation. (A) Heatmap depicting the expression of IL-21R as determined by Affymetrix GeneChip U133A gene chip in untreated NK cells and in NK cells stimulated for 12 hr with immobilized-IgG (100 μg/mL). Expression values were retrieved from the GEO database (GSE63038). Pixel density (highest values are red [+4], lowest are green [−4]) represents average hybridization signal intensity from eight donors pre- and post FcR-stimulation as detected by the probes for IL-21R, 219971_at and 221658_s_at. (B) Validation of IL-21R gene expression data by RT-PCR in untreated NK cells and NK cells exposed to immobilized-IgG (100 μg/mL) for 12 hr to stimulate the FcR. Each group depicts the mean fold increase in IL-21R expression in six donors ± SD. The asterisk (*) denotes p < 0.01 versus untreated NK cells.
![Figure 1. IL-21R gene expression and transcript levels are upregulated on NK cells following FcR stimulation. (A) Heatmap depicting the expression of IL-21R as determined by Affymetrix GeneChip U133A gene chip in untreated NK cells and in NK cells stimulated for 12 hr with immobilized-IgG (100 μg/mL). Expression values were retrieved from the GEO database (GSE63038). Pixel density (highest values are red [+4], lowest are green [−4]) represents average hybridization signal intensity from eight donors pre- and post FcR-stimulation as detected by the probes for IL-21R, 219971_at and 221658_s_at. (B) Validation of IL-21R gene expression data by RT-PCR in untreated NK cells and NK cells exposed to immobilized-IgG (100 μg/mL) for 12 hr to stimulate the FcR. Each group depicts the mean fold increase in IL-21R expression in six donors ± SD. The asterisk (*) denotes p < 0.01 versus untreated NK cells.](/cms/asset/063dcb45-c08b-4a16-9b47-968355f7d33f/koni_a_1312045_f0001_oc.gif)
Figure 2. The IL-21R is upregulated on NK cells following FcR stimulation in a time-dependent fashion. NK cells stimulated via the FcR by immobilized IgG were analyzed at varying time points for expression of IL-21R transcript by (A) RT-PCR and IL-21R protein by (B) immunoblot analysis, and (C) flow cytometry. (A) RT-PCR for IL-21R transcript in untreated NK cells and NK cells cultured in the presence of immobilized-IgG at the time points indicated. Data represent the mean fold increase in IL-21R expression in three donors ± SD. The asterisk (*) denotes p < 0.01 vs. all time points shown. (B) IL-21R expression at the protein level was confirmed by immunoblot analysis in primary NK cells and YT-CD16 cells at the time points indicated. The Ramos tumor cell line served as a positive control. The membranes were re-probed for β-actin to confirm equal loading. (C) IL-21R expression was measured by flow cytometry in resting NK cells and NK cells stimulated with immobilized-IgG. Cells were stained with anti-CD56-PE and anti-IL-21R-APC Abs at the time points indicated and fluorescence was compared to that obtained with an isotype control antibody. HeLa and Ramos acted as negative and positive controls, respectively. Percentages are reflective of dual positive populations (Q2). Each plot depicts the results from one representative donor. Results are representative of three normal donors tested.
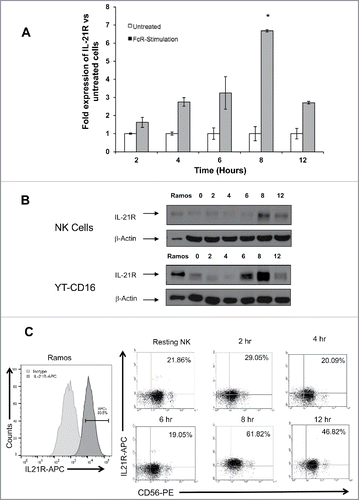
Figure 3. Upregulation of IL-21R on the NK cell surface is facilitated via the MAP kinase signaling pathway. (A) NK cells were stimulated with PMA or left resting, with the addition or absence of the ERK inhibitor U0126 and subjected to immunoblot analysis for phospho-ERK expression. (B) NK cells were stimulated for 8 hr with immobilized-IgG or left unstimulated in the presence of the ERK inhibitor U0126 (reconstituted in DMSO) and subjected to immunoblot analysis for IL-21R expression. The membranes were re-probed for β-actin to confirm equal loading. NK cells were isolated from two healthy donors.
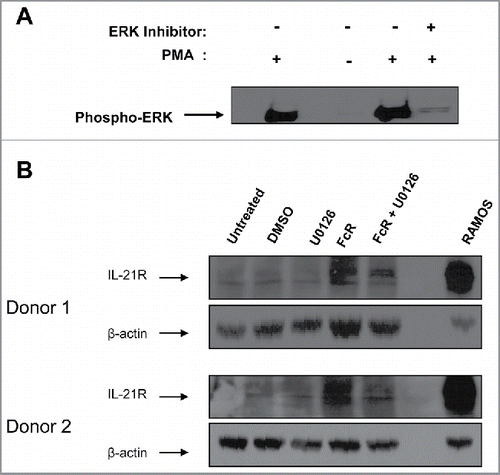
Figure 4. IL-21 treatment of FcR-stimulated NK cells enhances IL-21-mediated NK cell signal transduction. Resting NK cells and NK cells stimulated with immobilized-IgG for 8 hr were washed, rested for 1 hr, and then treated with PBS or 10 ng/mL of IL-21. 30 min later, NK cells were analyzed by flow cytometry and immunoblot analysis for (A) p (phospho)-STAT1 and (B) p-STAT3.
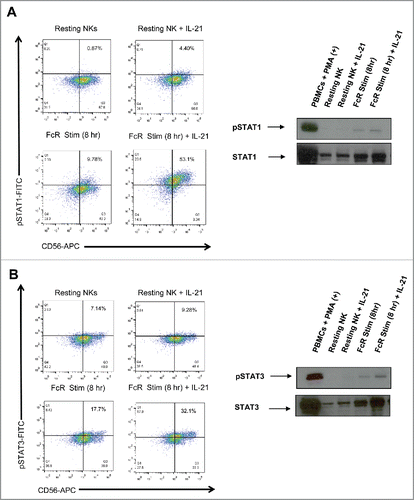
Figure 5. IL-21 treatment of FcR-stimulated NK cells enhances cytotoxicity, NK cell IFNγ production, and tumor cell apoptosis. (A) NK cells were left untreated or stimulated through their FcR for 8 h prior to incubation overnight with or without 10 ng/mL IL-21. The lytic activity of IL-21-activated NK cells was then assessed in a standard 4 h chromium release assay using K562 tumor cells as targets. The percentage of lysis was calculated as previously described. Graph depicts the results from one representative donor ± SD. Three normal donors were tested. The asterisk (*) denotes p < 0.05 vs untreated NK cells. (B) Untreated or 8 h FcR-stimulated NK cells were incubated with or without 10 ng/mL IL-21 and co-cultured with (100 μg/mL) trastuzumab-coated SK-BR-3 tumor cells. Supernatants were harvested at 48 h and analyzed for IFNγ by ELISA. Each graph depicts the mean production of IFNγ from three donors ± SD. The asterisk (**) denotes p < 0.01 and (*) denotes p < 0.05 vs. conditions shown. (C) Untreated or 8 h FcR-stimulated NK cells were incubated with or without 10 ng/mL IL-21 and co-cultured with (100 μg/mL) trastuzumab-coated SK-BR-3 tumor cells. Tumor cells were harvested at 48 h and stained for flow cytometry with anti-CD56-APC, propidium iodide, and anti-annexin V-V450. APC+ values were gated out to account for tumor cell apoptosis only. Percentages reported are of PE+/V450+ populations (Q2). Each graph depicts the results from three donors ± SD. The asterisk (*) denotes p < 0.05 vs. all conditions shown.
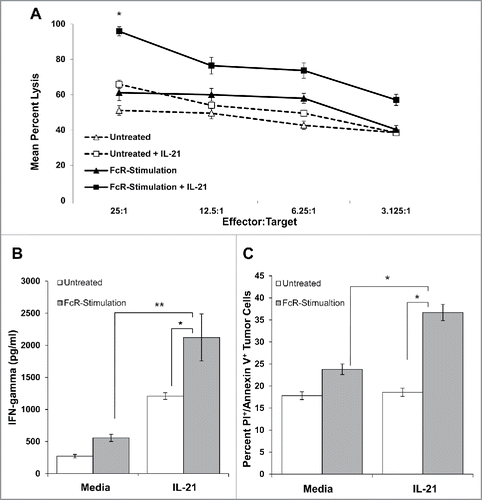
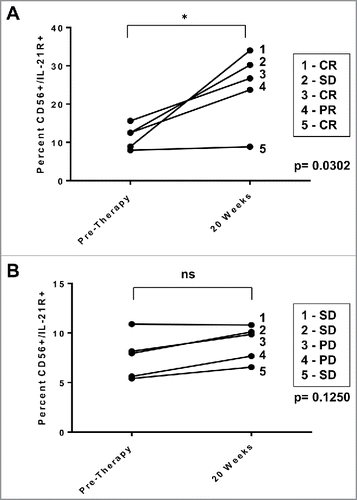
Figure 6. IL-21R expression is enhanced on NK cells in patients receiving monoclonal antibody therapy. NK cells from patients with HER2-positive breast cancer receiving trastuzumab-based therapy were analyzed for IL-21R expression. PBMC were obtained pre-therapy and at the 20 week time point. Total PBMC were stained with Abs specific for CD56 and IL-21R and analyzed by flow cytometry. (A) IL-21R expression in NK cells of patients with operable breast cancer who successfully received doxorubicin/ plus cyclophosphamide every 3 weeks × 4 cycles followed by weekly trastuzumab plus paclitaxel and exhibited clinical benefit (complete response, partial response, or stable disease = CR, PR, or SD). Time points are pre-therapy and at the 20 week time point at which time patients were receiving trastuzumab. Each data point is reflective of double positive populations determined by a gating strategy utilizing an isotype control antibody. The asterisk (*) denotes p = 0.0302 vs. pre-therapy. (B) IL-21R expression in NK cells of patients with metastatic breast cancer treated with trastuzumab and CpG oligodeoxynucleotides. Each data point is reflective of double positive populations determined by a gating strategy utilizing an isotype control antibody. Time points are pre-therapy and the 20 week mark at which time patients were receiving trastuzumab therapy. All patients eventually experienced progress on of disease. Percentages reported are of dual positive populations (Q2).