Figures & data
Figure 1. PBMC from cancer patients (n = 10) were stained and analyzed by flow cytometry for; A) Treg (CD3+CD4+CD127−CD25+FOXP3+ of lymphocytes), B) NK cell (CD3−CD56+ of lymphocytes), γδ T cell (CD3+γδ+), and Conventional T cell (CD3+FOXP3−) frequencies before and after ZA treatment. Statistical analyses were performed on pooled data using Student's t test.
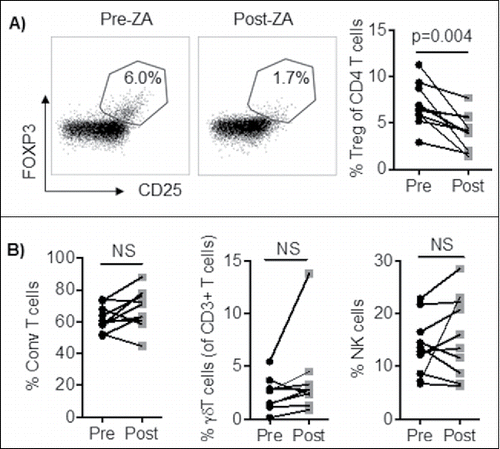
Figure 2. A) Enriched Treg from healthy blood donors were cultured with IL-2 and OKT3 for three days and analyzed for their proliferation by 3H-thymidine incorporation and viability by 7AAD and Annexin V. Statistical analyses were done on pooled data (n = 4) using non-parametric Wilcoxon matched-pair test. B) Heat-map or C) volcano plot of Differential Gene Expression (DEG) following RNA-sequencing of overnight cultured healthy blood donor Treg with IL-2, OKT3 in the presence or absence of ZA (n = 6). NS = not significant. The volcano plot shown illustrates the expression fold change (log2) vs. p-value (–log10). Single dots represent individual genes, where red dots indicate a p-value ≤ 0.05 and fold change of ≥1.3 while green dots indicate a p value ≤ 0.05 and a fold change of ≤ -1.3. D) The ranked DEG lists were used to perform GSEA using Gene Ontology annotated functions. Selected significantly altered gene set functions (p ≤ 0.05), showing the contribution of individual genes to the enriched gene sets in ZA treated Treg from healthy blood donor relative to untreated. E) The enrichment plot indicating the positive enrichment of genes associated with voltage gated calcium channel complex in ZA-treated compared to untreated healthy blood donor Treg.
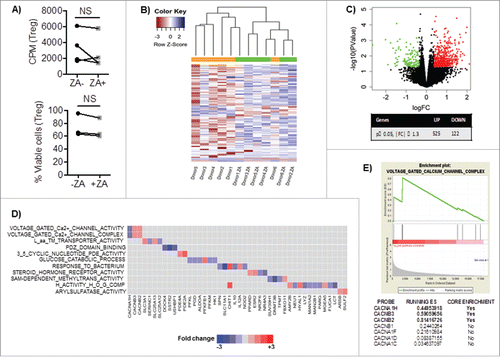
Table 1. Differential gene expression (DEG) analysis of gene sets that are significantly altered (p ≤ 0.05) in ZA-treated compared with untreated Treg and ranked by enrichment score.
Figure 3. A) Enriched Treg were pre-activated with IL-2 and OKT3 in the presence or absence of ZA overnight and stained for FOXP3 (green), NFAT (red) and the nuclear dye DAPI (blue). Cells were analyzed by confocal microscopy (20x) and images were analyzed using ImageJ 2.0. An enlarged image of one representative cell is shown in the bottom right corner. Representative images and accumulated data (n = 7) mean ± SEM are shown and statistical analyses were performed by the Student's t test. B) Enriched Treg were stimulated with IL-2 in the presence or absence of ZA for 3 days and analyzed for the expression of FOXP3 by flow cytometry. A representative histogram and pooled (n = 5 donors) data mean ± SEM are shown and statistical analyses were performed by Wilcoxon test.
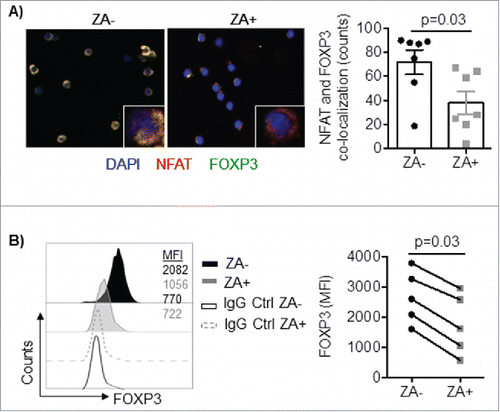
Figure 4. A) Enriched Treg from healthy blood donors (n = 6) were stimulated with IL-2 in the presence or absence of ZA for 3 days and analyzed for the expression of CD25 by flow cytometry. A representative histogram and accumulated data mean ± SEM are shown and statistical analysis was performed by Wilcoxon test. Each symbol represents a distinct healthy individual. B) Enriched Treg from healthy blood donors (n = 3) were stimulated with and without IL-2 in the presence or absence of ZA for 15, 30, and 90 minutes and analyzed for the expression of phosphorylated STAT5 by flow cytometry. Representative histograms and accumulated data mean ± SEM are shown and statistical analysis was performed by Two-way ANOVA. **p = 0.004, *p = 0.01. C) Enriched or sorted Treg (n = 6) were overnight stimulated with IL-2 and OKT3 in the presence or absence of ZA and stained for total TGFβ. A representative histogram and accumulated data are shown and statistical analyses were performed by Wilcoxon test.
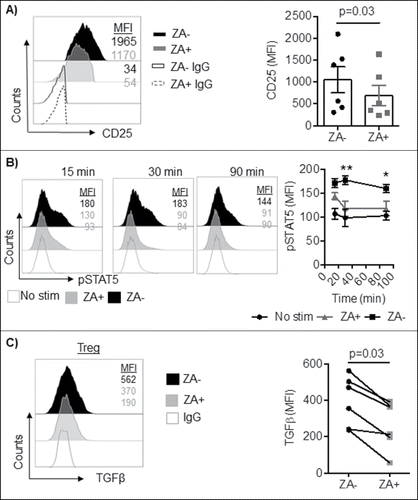
Figure 5. Treg were pretreated with OKT3, IL-2, +/− ZA, and co-cultured with CD4+ T cells and HLA-DR+ (1:1) or NK cells at different ratios (1:1-1:8) in the presence of OKT3 or IL-2 for 3 and 4 days respectively. A) Cell proliferation was measured by 3H-thymidine incorporation. Representative data are shown of three independent experiments mean ± SEM and statistical analyses were performed using Two-way ANOVA to analyze statistical differences between all groups. B) Following 3 days of preactivation with IL-2 and OKT3 in the presence or absence of ZA, Treg were co-cultured (1:2) with Her2-specific chimeric antigen receptor (CAR)-transduced T cells or NK cells and co-incubated with chromium 51Cr labeled Her2-expressing SKOV3 or K562 target cells respectively at different E:T ratio (2:1-0.5-1) for 18 hours. Representative data are shown from three independent experiments mean ± SEM and statistical analyses were performed by Two-way ANOVA to analyze statistical differences between all groups.
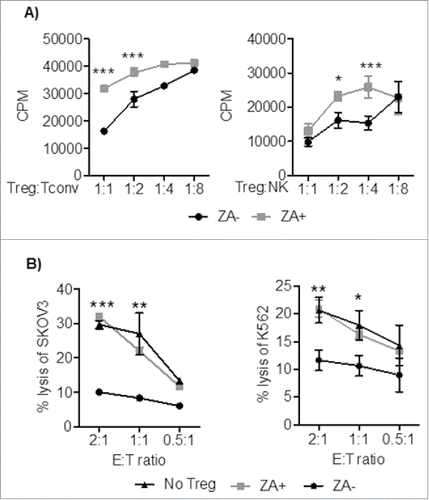
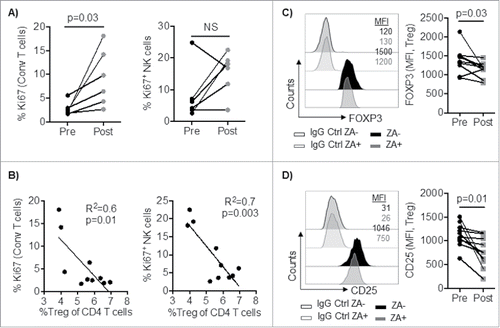
Figure 6. A) PBMC from cancer patients (n = 6) were stained and analyzed by flow cytometry for conventional (CD3+FOXP3−) T cell and NK cell proliferation (Ki67-positive cells) pre and one week post ZA administration. B) Correlation between percent circulating Treg and NK and T cell proliferation (Ki67). Statistical analyses were performed on pooled data using A) Wilcoxon test, and B) Pearson's correlation. PBMC from cancer patients (n = 10) were stained and analyzed by flow cytometry for the expression of C) FOXP3 and D) CD25. Representative histograms and pooled data are shown and Statistical analyses were performed using Student's t test.