Figures & data
Table 1. Risk of postoperative recurrence in stage III colorectal cancer by TAM densities, clinical-pathological features, and 5-fluorouracil (5FU) adjuvant therapy.
Table 2. Outcome predictors in 5-fluorouracil-treated patients with stage III colorectal cancer (Cox univariate analysis).
Figure 1. Disease-free survival (DFS) in stage III colorectal cancer, by TAM-density quartiles. Kaplan-Meier Curves. Patients were stratified by quartile distribution of TAMs densities at the invasive front of their primary tumor (PT-TAMs, upper panels) or of metastatic lymph-nodes (LN-TAMs, lower panels). Higher densities of both PT-TAMs and LN-TAMs were significantly associated with better disease-free survival in patients treated with 5-fluorouracil (left panels), but not in the subset of untreated patients (right panels). Also higher densities of tumor-associated neutrophils (PT-TANs) were weakly associated with the survival of 5-fluorouracil -treated patients (see Fig. S1). P values are for Log-Rank test.
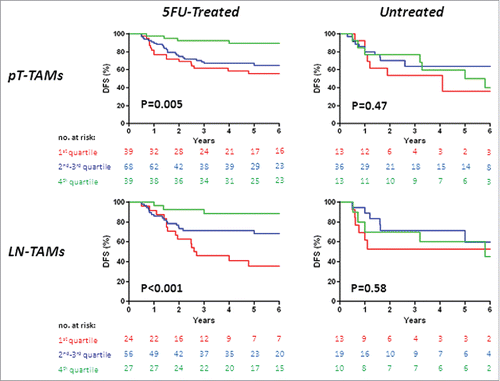
Figure 2. Disease-free survival (DFS) in 5-fluorouracil-treated patients with stage III colorectal cancer, by high/low TAMs. Kaplan-Meier Curves. Patients were classified by high/low density of TAMs, measured at the invasive front of their primary colorectal cancer (PT-TAMs) or of metastatic lymph-nodes (LN-TAMs), and defined by optimal cut-offs at receiver operator characteristic (ROC) curves (Fig. S2). P values are for Log-Rank test. Also high densities of intra-tumoral neutrophils (PT-TANs) were weakly (p = 0.04) associated with better disease-free survival (Fig. S3). The association of high PT-TAMs with better disease-specific survival was confirmed in the external validation set (Fig. S4).
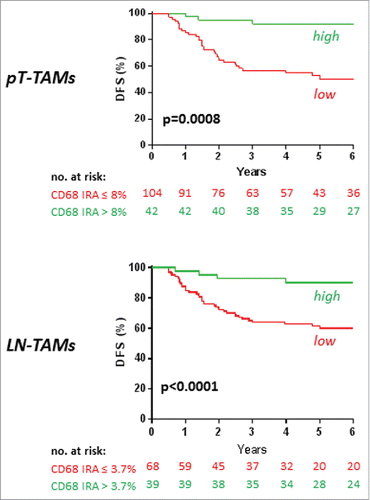
Figure 3. Chemotherapy and macrophage synergism in cytotoxic function in vitro. A) Facs plots of SW480 colorectal cancer cells co-cultured with un-polarized (M0) or macrophages polarized toward a cytotoxic phenotype by IFNg/LPS stimulation (M1). Tumor cell death was evaluated by Annexin V/7-AAD staining. 24-hour 5-fluorouracil treatment on colorectal cancer cells alone did not significantly increase tumor cell death compared with untreated cells (top). Macrophages induced detectable tumor cell death, which was further enhanced by treatment with 5-fluorouracil (bottom). One representative of 5 experiments is shown. B) Histograms representative of 3 independent experiments performed with 2 MSS cell lines (SW480 and HT29): cytotoxicity of M1 macrophages toward colorectal cancer cells (*; P ≤ 0.05) was significantly increased by the addition of 5-fluorouracil in the 2 microsatellite stable cell lines, SW480 (**; P ≤ 0.005) and HT29 (***P ≤ 0.001). Histograms show means ± standard error. C) Culture of macrophages with supernatant of 5-fluorouracil-treated colorectal cancer cells (MfCM) significantly increased macrophage cytotoxicity (right) compared with control macrophages (left) or macrophages cultured with supernatant of control colorectal cancer cells (MfCT) (middle). One representative of 2 experiments is shown. D) Histograms representative of 2 independent experiments (***; P ≤ 0.001; **; p ≤ 0.01). E) Effect of chemotherapy on macrophage polarization markers. 5-Fluorouracil treatment of M0 macrophages increased the expression of the M1-marker HLA-DR, while the M2-marker CD163 did not shown any change. F) Effect of chemotherapy on macrophages-M1 cytotoxicity. TRAIL and TNFa expression was measured by ELISA in cellular extracts from macrophages stimulated with LPS/IFNg and subsequently exposed to 5-fluorouracil for 24 hours. One representative of 2 experiments is shown. Bars represent means ± standard error. (*: p <0.05; **: p ≤ 0.01).
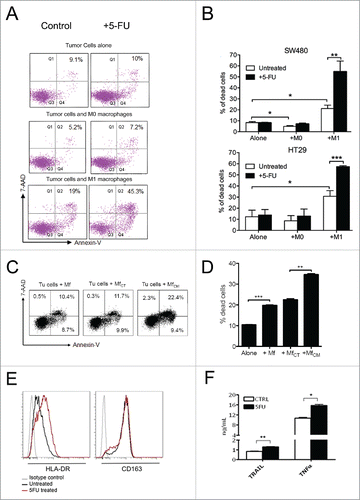
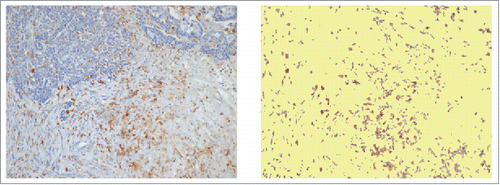
Figure 4. Immunostaining of CD68+ TAMs at the invasive margin of primary colorectal cancer (A) and of metastatic lymph-nodes (B). Examples of tissue areas with variable extent of CD68-immunoreative area (% IRA in white boxes).
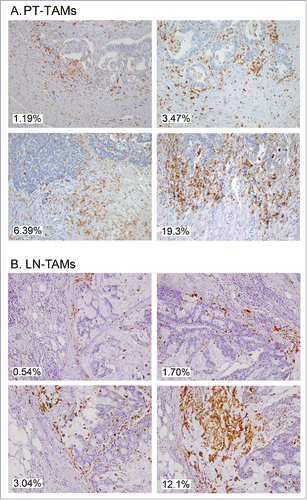
Table 3. Cox multivariate analysis of TAM and TAN densities, and other pathological features predicting the outcome of stage III colorectal cancer treated with 5-fluorouracil adjuvant therapya
Figure 5. Capture of immune reactive areas at image analysis. Example of computer assisted selection of immune reactive areas spots by red green and blue (RGB) color segmentation of the original digital microphotograph. Immune-reactive area, brown. Not stained tissue, yellow. The percent ratio between immune reactive areas and total area was automatically calculated.