Figures & data
Figure 1. Increased mortality and tumorigenesis in BLT1−/−ApcMin+ mice. (A) Kaplan-Meier plot of survival from ApcMin/+ (n = 27) (blue), BLT1+/−ApcMin/+ (n = 29) (green), and BLT1−/−ApcMin/+ mice (n = 29) (red) are shown. Difference in survival of ApcMin/+ mice compared with other 2 strains was determined to be significant by the Mantel-Haenszel/Log-rank test (P< 0.0001). (Mean survival for ApcMin/+ (156 days), BLT1+/−ApcMin/+ (126 days), and BLT1−/−ApcMin/+ (102 days). (B) Severe anemia in BLT1−/−ApcMin/+ mice. Hematocrit values were determined for the indicated mice at the age of ∼110 d. Statistical analysis was performed using Mann-Whitney U test (*** = P < 0.001). (C-F) Increased intestinal tumor burden in BLT1−/−ApcMin/+ mice. Total number of polyps in the small intestine (C) and colon (D) were quantified by stereoscopic microscopy in age matched (100–110 d old) ApcMin/+ (n = 18) and BLT1−/−ApcMin/+ (n = 12) mice using longitudinally opened tissue sections. (E) The size distribution of colon tumors in ApcMin/+ (blue bar) and BLT1−/−ApcMin/+ (red bar) was measured. There was a significant increase in the large sized tumors (> 2 and >3 mm) in BLT1−/−ApcMin/+ when compared with ApcMin/+. Statistical analysis was performed using Mann-Whitney U test. Error bars, ± SEM. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. (F) Representative cross section images of colons stained with Hematoxylin and Eosin (H&E) shows increased number and size of tumors in BLT1−/−ApcMin/+ compared with ApcMin/+. Images were captured using Aperio Image scope, Aperio Technologies Inc.
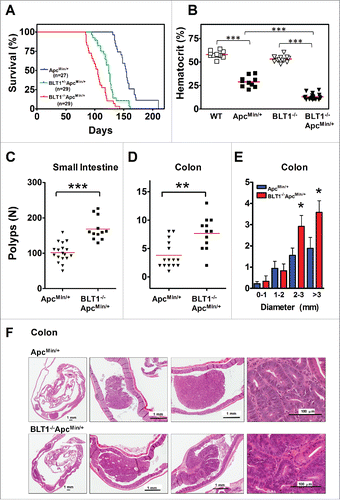
Figure 2. Analysis of β-catenin localization, proliferation and apoptosis in BLT1−/−ApcMin/+ tumors. (A) IHC analysis of β-catenin staining of colon adenomas of ApcMin/+ and BLT1−/−ApcMin/+ mice. The polyps from the BLT1−/−ApcMin/+ mice showing more intense nuclear localization of β-catenin relative to the colon polyps ApcMin/+. (B) 5-bromo-2-deoxyuridine (5-BrdU) injected into mice 2 hrs before sacrificing the mice. The cross section of colons stained with BrdU antibody. Immunohistochemical (IHC) analysis of 5-BrdU-incorporation showed a significant increase in the number of proliferating cells in colonic polyps of BLT1−/−ApcMin/+ mice. (C) Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) stain performed on colons of ApcMin/+ and BLT1−/−ApcMin/+ mice. The number of TUNEL positive apoptotic cells are significantly decreased in BLT1−/−ApcMin/+ tumors compared with ApcMin/+ mice. Statistical analysis was performed using Mann-Whitney U test. Error bars, ± SEM and ***, P < 0.001.
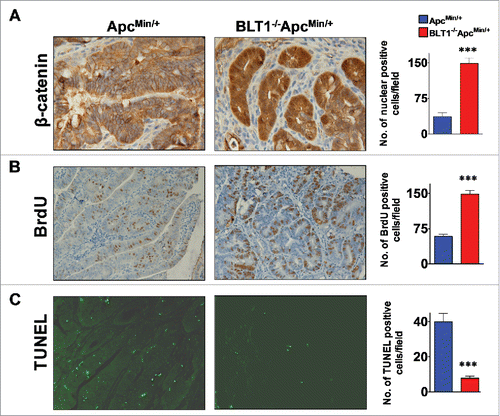
Figure 3. BLT1−/− mice are more susceptible to DSS induced colitis and AOM/DSS induced colon tumorigenesis. Wild type (WT) and BLT1−/− mice received 3% DSS in drinking water ad libitum for the duration of the experiment. (A) The bodyweights of wild type (n = 10) and BLT1−/− (n = 8) mice were measured every 2 d for 2 weeks using weight at Day 0 of the experiment as 100% for calculating weight loss. (B) The mice were killed at day 14 and colon length of BLT1−/− (n = 10) was significantly reduced compared with wild type mice (n = 11). (C) Tissue bacterial loads of DSS treated Wild type (n = 8) and BLT1−/− (n = 8) mice. The spleen, liver, mesenteric lymph nodes, and the colon were aseptically removed and each tissue was then homogenized in sterile 1X PBS and was serially diluted and plated on tryptic soy agar plates and incubated for 24 hrs at 37°C. Colonies were counted and total CFUs were determined based on the total volume of the specimens. (D-E) Increased incidence of azoxymethane (AOM)/DSS induced colon cancer in BLT1−/− mice. The number of polyps in colon increased in BLT1−/− mice treated with AOM/DSS (D), and representative colon images of AOM-DSS treated BLT1−/− and BLT1+/+ mice are shown (E). The red arrows point to visible tumors. Statistical analysis was performed using the Mann-Whitney U test. Error bars, ± SEM. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
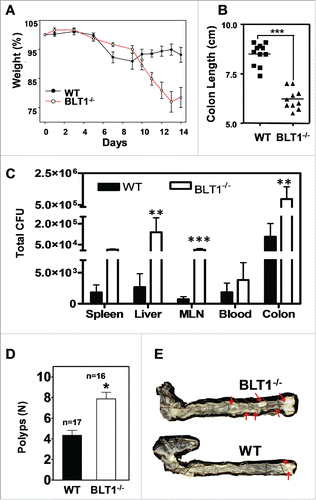
Figure 4. Increased inflammatory and decreased host defense markers in BLT1−/−ApcMin/+ tumors. (A-B). The mRNA levels were measured by qRT-PCR using SyBR green dye (Applied Biosystems) from size matched colon tumors of ApcMin/+ and BLT1−/−ApcMin/+ mice (105–110 d age old). Data are representative of tumors/tissues isolated from at least 5 different mice for each genotype. (A). mRNA levels of inflammatory mediators (TNF-α, IL-6, CXCL1 and COX2) are significantly increased in colonic tumors of BLT1−/−ApcMin/+ compared with ApcMin/+ mice. Statistical analysis was performed using the Mann-Whitney U test. Error bars, ± SEM. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (compared with ApcMin/+). (B). Immunohistochemical analysis indicates increased expression of COX2 in the colon tumors of BLT1−/−ApcMin/+ compared with ApcMin/+ mice. (C) The relative mRNA levels of host defense proteins (Ang, Ang 4, IDO and Reg3γ) were reduced in BLT1−/−ApcMin/+ compared with ApcMin/+ mice colon tumors. (D) Western blot analysis of angiogenin expression in colons and colon tumors of BLT1−/−ApcMin/+ compared with ApcMin/+.
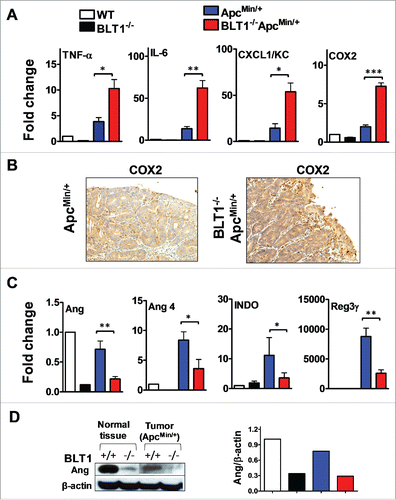
Figure 5. Germ-free BLT1−/−ApcMin/+mice were protected from colon cancer. (A). Gross appearance of longitudinally opened colons from BLT1−/−ApcMin/+mice raised and maintained in specific pathogen free (SPF) and germ free (GF) conditions. (B). BLT1−/−ApcMin/+ mice (n = 12) maintained in germ free facility are highly protected from developing colon tumors compared with SPF facility maintained BLT1−/−ApcMin/+ mice (n = 12). Oral gavage of fecal material from the SPF BLT1−/−ApcMin/+ mice to germ-free BLT1−/−ApcMin/+ mice (GF_FD; n = 8) resulted in colon tumor development in these mice. (C). Longitudinally opened distal small intestines of BLT1−/−ApcMin/+mice maintained specific pathogen free (SPF) or germ free (GF). (D). BLT1−/−ApcMin/+ mice maintained in germ free or SPF facility developed similar number of small intestinal tumors. E. The representative H and E stained swiss roll sections of small intestine (distal) (left panel), colon (right panels) of the BLT1−/−ApcMin/+ mice maintained at GF and SPF facilities. Images were captured using Aperio Image scope.
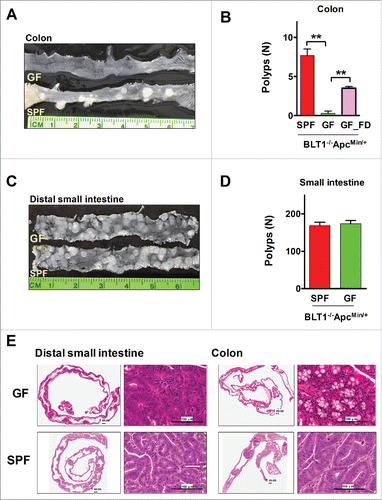
Figure 6. MyD88 acts downstream of BLT1 in promoting intestinal cancer in ApcMin/+ mice. (A). BLT1/MyD88 double knockout mice are susceptible to lethal neonatal infections. Percent survival of the offspring from BLT1−/−MyD88+/− cross (total of 15 litters from 8 different breeding pairs at day 15). Treatment of BLT1−/−MyD88+/− females during gestation and postpartum with a broad-spectrum antibiotic Baytril (enrofloxacin at 165 mg/L in drinking water that gave a daily dosage of ∼25 mg/kg) completely reversed the early lethality of BLT1−/−MyD88−/− pups. (B). Double deficient (BLT1−/−MyD88−/−) mice are smaller compared with the littermate heterozygous mice (BLT1−/−MyD88+/−). (C) H&E stained cross section images of lung from (BLT1−/−MyD88−/− mice (top panel). The bacterial infection was detected by FISH analysis (lower panel) with Eub338-Cy3 probe (red) and nuclei of lung parenchyma stained with DAPI (blue). (D-I). The BLT1−/−MyD88−/−ApcMin/+ and littermate control animals were generated and maintained on Baytril water. The hematocrits (D), number of small intestine polyps (E) and colon polyps (F) were evaluated at 110 d age-old mice. The frequency of small intestinal tumors (G) and size ranges of tumors in both small intestine (H) and colons (I) were analyzed. The overall tumor burden in mice lacking MyD88 significantly reduced in both BLT1+/+ and BLT−/−ApcMin/+ context indicating that MyD88 acts downstream of BLT1 in promoting intestinal cancer. Statistical analysis was performed using the Mann-Whitney U test. Error bars, ± SEM. **, P < 0.01; and ***, P < 0.001.
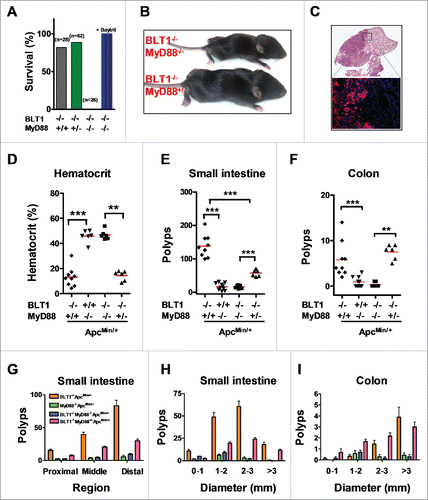
Figure 7. Modulation of gut microbiota and barrier dysfunction in BLT1−/−ApcMin/+. The gut microflora (fecal bacteria) was identified by sequencing 16 S rRNA gene using Roche 454 sequencer from indicated genotype (WT, n = 5; BLT1−/−, n = 5; ApcMin/+,n = 8; BLT1−/−ApcMin/+ n = 10; MyD88−/−ApcMin/+ (n = 5); BLT1−/−MyD8−/−ApcMin/+ (n = 5)). The 16 S rRNA gene (v1-v3 regions) sequences were uploaded on to QIIME pipe line (http://qiime.sourceforge.net/#). The distribution of phylum (A) and genus levels (B) among genotypes were identified. Genus level analysis suggested significant increase in Akkermansia muciniphila. (C). The relative amounts of Akkermansia muciniphila from the fecal contents of indicated mice was quantified using real time PCR as described in methods. Statistical analysis performed using unpaired t-test. ** P< 0.01, *P< 0.05. (D). The α diversity is measured using qiime pipeline. (E). Increased gut permeability and decreased tight junctional proteins in tumor bearing mice. The FITC-dextran (MWav = 4000; FD4) at 80 mg/mL (in 1X PBS) was administered by oral gavage (600 mg/kg bodyweight) to fasted mice. The fluorescence of FITC was measured in plasma after 2 hrs to estimate amount of FD4 leaked into blood and percent FD4 calculated using WT as base line. FD4 was significantly increased in BLT1−/−ApcMin/+ compared with ApcMin/+(*P< 0.05) and in ApcMin/+ compared with WT (*P< 0.05). (F). The mRNA levels of Cldn 5 and Jam C proteins was measured by RT-PCR using SyBr green. The mRNA levels were significantly reduced in tumors of ApcMin/+ and BLT1−/−ApcMin/+ mice.
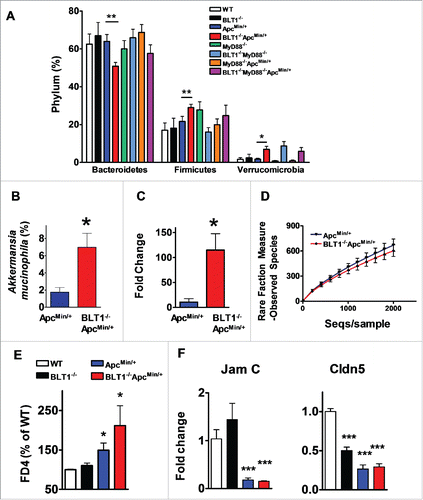
Figure 8. Interplay of BLT1 and MyD88 Signaling in Intestinal tumor development: Adenoma induced barrier dysfunction in ApcMin+ mice induces tumor promoting inflammation. Absence of BLT1 amplifies tumor initiated microbial dysbiosis leading to both bacteria (germ-free) and MyD88 dependent inflammation that promotes tumor progression.