Figures & data
Figure 1. (A) Cartoon illustrating the model system of the co-culture (tumor cells/T-cells) with the bispecific antibody (scDbFAPxCD3) and the costimulatory fusion proteins (B7.1-DbFAP, scFvEDG-4–1BBL/OX40L). FAP: fibroblast activation protein, EDG: endoglin, TCR: T cell receptor. Proliferation of (B) CD4+ and (C) CD8+ T cell subpopulations in response to stimulation by the bispecific antibody and costimulatory antibody-fusion proteins. HT1080-FAP cells were co-cultured with CFSE-labeled PBMCs in presence of recombinant protein combinations for 6 d. Naïve (CD45RA+,CCR7+)(TN), central memory (CD45RA−,CCR7+)(TCM), effector memory (CD45RA−,CCR7−)(TEM) and effector (CD45RA+,CCR7−)(TE) CD4+ and CD8+ T cells were identified and proliferation measured by flow cytometry. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistic comparison refers either to the effect of the scDb alone or in case of costimulatory fusion protein combinations to the highest individual costimulatory effect. Gray background points out the combination of 2 costimulatory fusion proteins.
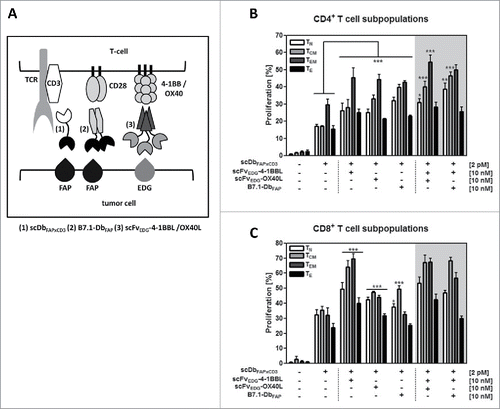
Figure 2. Expression and activity of endogenous IL-10 and TGF-β in the HT1080-FAP/PBMC co-culture setting. (A) Release of IL-10 and TGF-β into the co-culture supernatant (HT1080-FAP/PBMC unstimulated or stimulated) was determined after 24 h by sandwich-ELISA. (B) Immunosuppressive activity of IL-10 and TGF-β was demonstrated by blockade with antagonistic antibodies. T cells were stimulated via scDbFAPxCD3 in the HT1080-FAP/PBMC co-culture setting +/− anti-IL-10R mAb (10 µg/ml) and anti-TGF-β mAb (5 µg/ml), respectively. IFN-γ release was measured after 48 h by sandwich-ELISA. (C) Stimulation of T cells by the combinatorial setting of bispecific antibody and costimulatory fusion proteins +/− blocking IL-10 and TGF-β activity with 10 µg/ml anti-IL-10R mAb and 5 µg/ml anti-TGF-β mAb, respectively. IFN-γ release was measured after 48 h by Sandwich-ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.
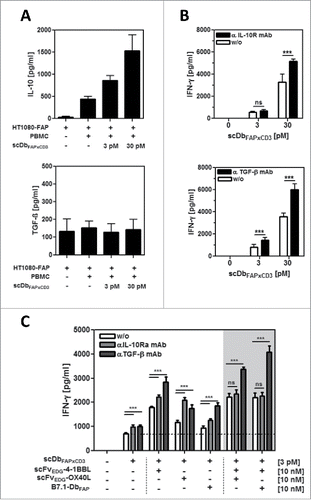
Figure 3. Effect of exogenous IL-10 and TGF-β on PBMC stimulation by fusion protein combinations in the HT1080-FAP/PBMC co-culture. HT1080-FAP cells were co-cultured with PBMCs +/− 10 µg/ml recombinant IL-10 or TGF-β in presence of (A) bispecific antibody titrated +/− 10 nM scFv-4–1BBL, (B) 5 pM bispecific antibody +/− costimulatory fusion protein titrated and (C) 5 pM bispecific antibody +/− 10 nM costimulatory fusion protein single or combined. After 48 h, IFN-γ concentration in the supernatant was measured in ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistic comparison refers to the corresponding effect of the scDb alone. Gray background points out the combination of 2 costimulatory fusion proteins.
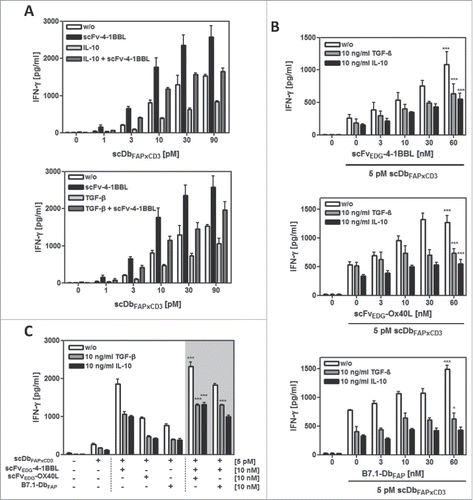
Figure 4. IDO expression and activity in the HT1080-FAP/PBMC co-culture setting. (A) IDO expression in HT1080-FAP cells was detected via flow cytometry by intracellular staining with FITC-conjugated anti-IDO mAb after 24 h co-culture with stimulated (0,2 µg/ml anti-CD3-mAb) (gray line) or unstimulated (black line) PBMC. Isotype control (dotted line), cells only (gray filled). (B) IDO activity inhibits PBMC proliferation. CFSE-labeled PBMCs in co-culture with HT1080-FAP cells were stimulated with 0,1 µg/ml cross-linked α-CD3 mAb for 6 d in presence or absence of 5 nM INCB024360. Proliferation was measured by flow cytometry. (C) Blocking IDO activity in the HT1080-FAP/PBMC co-culture enhances the stimulation induced by the combinatorial setting of recombinant fusion proteins. HT1080-FAP cells were incubated with combinations of bispecific antibody and costimulatory fusion proteins in presence of 5 nM INCB024360. CFSE-labeled PBMCs were added and after 6 d proliferation of PBMCs measured by flow cytometry. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.
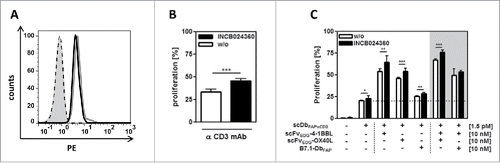
Figure 5. Costimulatory potential of the combinatorial fusion protein setting in presence of Tregs. HT1080-FAP cells were incubated with the recombinant fusion proteins for 1 h. (A,B) Freshly isolated CD4+CD25−T cells and CD4+CD25+ Tregs were added and after 24 h activation measured by (A) CD69 expression via flow cytometry and (B) IL-10 release by ELISA. (C) Suppressive activity of activated and expanded Tregs on CD4+ T cells was assayed by adding them at the TE: Treg ratios of 4:0, 4:1 and 4:4. After 48 h supernatant was harvested and IFN-γ measured in ELISA. Graphics show mean ± SD, n = 3. Gray background points out the combination of 2 costimulatory fusion proteins.
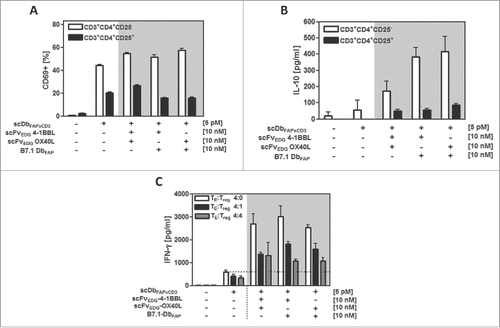
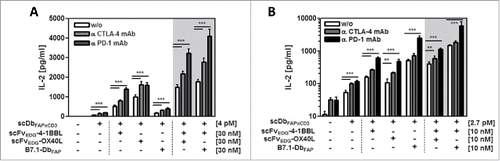
Figure 6. Stimulation of resting (A) and activated (B) T cells by the combinatorial fusion protein setting in presence of CTLA-4 and PD-1 checkpoint inhibitors. HT1080-FAP cells were incubated with the combinatorial setting of bispecific antibody and costimulatory fusion proteins for 1h, followed by the addition of 10 µg/ml anti-CTLA-4 or anti-PD-1 mAb, respectively. Then, resting or previously activated (0.1 µg/ml cross-linked anti-CD3 mAb for 6 days) PBMCs were added. After 24 h IL-2 concentration was determined by ELISA. Graphics show mean ± SD, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Gray background points out the combination of 2 costimulatory fusion proteins.