Figures & data
Figure 1. CD20-directed blocking of CD47-SIRPα interaction by RTX-CD47 (A) Schematic representation of RTX-CD47 comprising a CD20-targeting scFv derived from rituximab genetically fused to a CD47-blocking scFv and lacking an Fc domain. (B) RTX-CD47 selectively binds to CD20posCD47pos cell lines and not to CD20negCD47pos cell lines. Binding of RTX-CD47 to the cells was determined by flow cytometry using an HA tag antibody. (C) RTX-CD47 binding to Ramos CD20pos/CD47pos cells in the presence or absence of CD20-blocking antibody RTX (5 μg/mL) and/or CD47-blocking antibody B6H12 (5 μg/mL). Binding of RTX-CD47 could only be blocked by simultaneously adding excess amounts of CD20- and CD47-competing MAbs. (D) SIRPα-Fc binding to CD47 was blocked by RTX-CD47 on CD20/CD47 double positive cells (WIL2S and Z138) and not on CD20negCD47pos (SEM and DLD1). Binding of SIRPα-Fc to the cell surface of the cells was determined by flow cytometry using human recombinant SIRPα-Fc (5μg/ml) followed by staining with mouse anti-SIRPα and Alexa Fluor 488-conjugated goat anti-mouse IgG. (E) Bar graph displaying the MFI of the SIRPα-Fc binding as in (D), with the addition of a condition in which SIRPα-Fc binding was restored by an excess of the CD20-competing antibody rituximab (5 μg/ml) prior to RTX-CD47 binding on CD20pos/CD47pos WIL2S tumor cells.
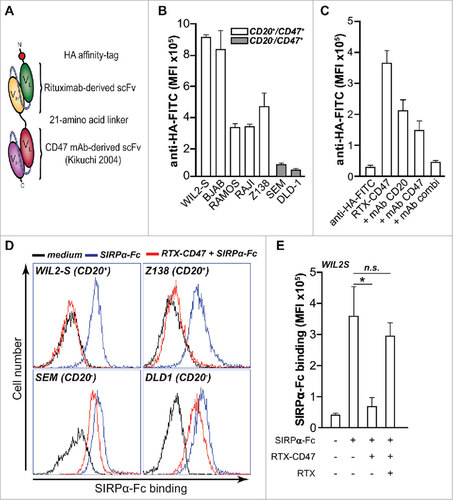
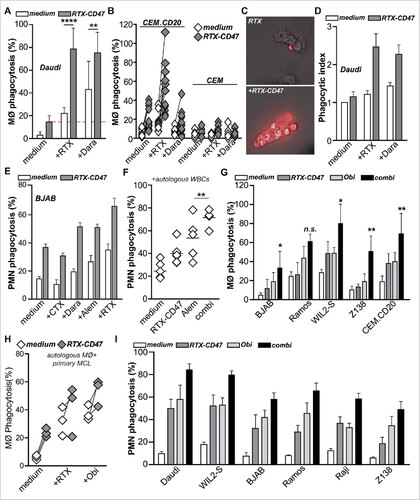
Figure 2. RTX-CD47 has single-agent pro-phagocytic activity towards CD20pos cancer cells. (A) Representative photos for macrophage phagocytosis assay with Daudi cells treated with RTX-CD47. RTX-CD47 induced macrophage-mediated phagocytosis of 4 out of the 6 CD20pos cancer cell lines. (B) RTX-CD47 triggered phagocytosis of primary patient-derived malignant B cells by autologous macrophages. (D) RTX-CD47 enhanced PMN-mediated phagocytosis of CEM.CD20 cells but not of parental CEM cells. (E) RTX-CD47 induced phagocytosis of all CD20/CD47 double positive cell lines in this cell panel and had no phagocytic activity on CD20negCD47pos cancer cell lines. (F) RTX-CD47 triggered granulocyte-mediated phagocytosis of primary patient-derived B malignant cells. (G) Phagocytosis induced by RTX-CD47 on WIL2 S cells is blocked by the addition of RTX F(ab')2 (5 µg/ml).
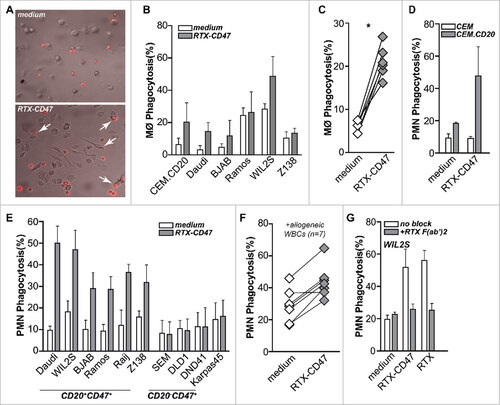
Figure 3. RTX-CD47 enhances ADCP by therapeutic anticancer antibodies. (A) RTX-CD47 synergized with therapeutic mAb in mediating phagocytosis of Daudi cells by macrophages. (B) RTX-CD47 enhanced (RTX mediated) phagocytosis of CEM.CD20 cells but not of parental CEM cells. (C) Representative photos of single macrophages engulfing V450-labeled target cells upon combined treatment with RTX-CD47 and RTX. (D) Quantification of phagocytic index from (A) showing average number of engulfed tumor cell per macrophage. (E) Combined treatment with RTX-CD47 and therapeutic antibodies (cetuximab, daratumumab, alemtuzumab (5 ng/ml) and rituximab (10 ng/ml)) significantly augmented phagocytosis levels BJAB cells. (F) Combined treatment with RTX-CD47 and alemtuzumab (1 µg/ml) significantly augmented phagocytosis levels of primary b-cell malignancies. (G) RTX-CD47 treatment potentiated macrophage-mediated phagocytosis of CD20pos cancer cell lines by obinutuzumab. (H) RTX-CD47 potentiated obinutuzumab mediated phagocytosis of primary patient-derived MCL cells by autologous macrophages. (I) RTX-CD47 treatment potentiated PMN- mediated phagocytosis of CD20pos cancer cell lines by obinutuzumab.