Figures & data
Table 1. Peptide sequences, aa coordinates and screening pools, and murine homology.
Table 2. Healthy donor details.
Figure 1. Screening IFNγ responses to peptide pools. Transgenic mouse strains with human DR4 (A) or DR1/HHDII (B) and parental C57Bl/6 (C and D) mice were used to screen IFNγ responses to peptides. Mice were immunized with pools of 4–6 non-overlapping human citrullinated ENO1 peptides. Ex vivo responses to stimulation with human and mouse (Mo) equivalent citrullinated peptides were assessed by IFNγ ELISpot. Media only responses were used as a negative control. For each pool n = 3. Statistical significance of peptide compared to media responses for each pool was determined by ANOVA with Dunnett's post-hoc test # p<0.05, ## p<0.01, ### p<0.001.
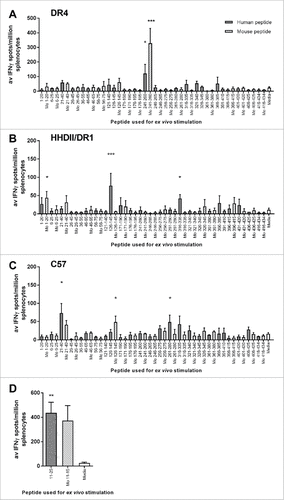
Figure 2. Human ENO1 241–260cit253 and 11–25cit15 peptide induces strong IFNγ responses. Transgenic HLA-DR4 or parental C57Bl/6 mice were immunized with human (Hu) ENO1 241–260cit253 or Hu ENO1 11–25cit15 peptide, respectively. Ex vivo ELISpot was used to determine the IFNγ responses to citrullinated (cit) peptide and wild type (wt) peptides (A). Responses were also determined after immunization with Mouse (Mo) equivalent peptides (B). Further, IFNγ responses after ex vivo addition of anti-CD4 or anti-CD8 blocking antibodies were determined (C) and ex vivo Granzyme B production in response to peptides determined by ELISA (D).
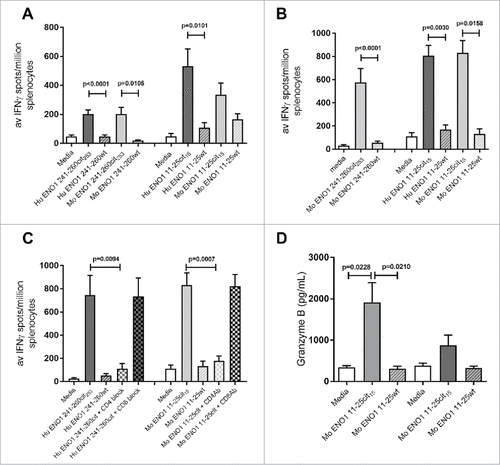
Figure 3. Immunization with citrullinated ENO1 peptides provides an in vivo survival advantage in anti-tumor studies. ENO1 Westerns blots show ENO1 protein expression in cancer cell lines, B16F1 (melanoma), ID8 (Ovarian cancer), TrampC1 (Prostate cancer), Pan02 (Pancreatic ductal adenocarcinoma), LLC/2 (Lung Carcinoma), RTLCL (Lymphoblastoid cell line) and in HeLa cells (A). HLA-DR4 mice were challenged with subcutaneous implant on HLA transgenic B16-DR4 (B), LLC2-DR4 (C), PAN02-DR4 (D) tumor cell lines on day 1. Survival is shown for unimmunized control animals and animals immunized with ENO1 241–260cit253 peptide on day 4, 11 and 18. Mice were also challenged with parental B16F1 (E) or LLC2 (F) cell lines before immunization as above.
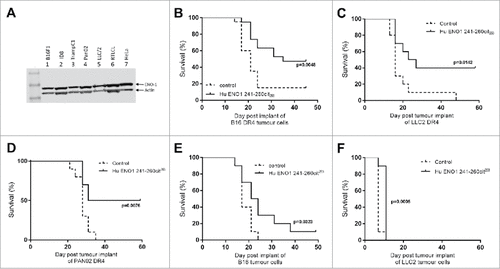
Figure 4. Anti-tumor advantage of immunized mice is seen in models with IFNγ-inducible MHC-II. Transgenic HLA-DR4 mice were challenged with B16 tumor with IFNγ inducible DR4 (B16-iDR4). Survival was determined after immunization with Hu ENO1 241–260cit253 on day 4, 11 and 18 (A). Surviving mice from the immunized group were rechallenged with the same tumor cell line at day 42 post initial tumor implant (indicated by arrow). Survival data for rechallenged mice and a previous unchallenged control group are shown (B). C57bl/6 mice were also challenged with parental B16F1 implanted subcutaneously on day 1 and survival was assessed in unimmunized control mice or mice immunized with ENO1 11–25cit15 peptide (C). In a separate experiment, mice implanted with the B16-iDR4 cell line were immunized with the mouse ENO1 241–260cit253 or 241–260 wt peptides (D). For tumor studies, each group had n = 10, significant p values are shown.
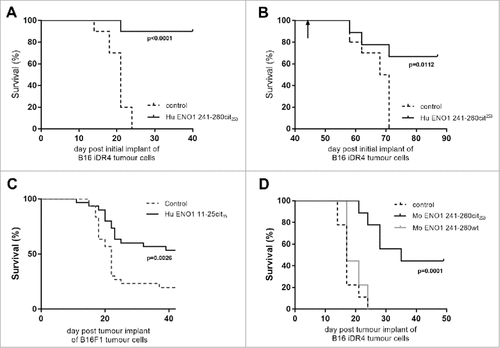
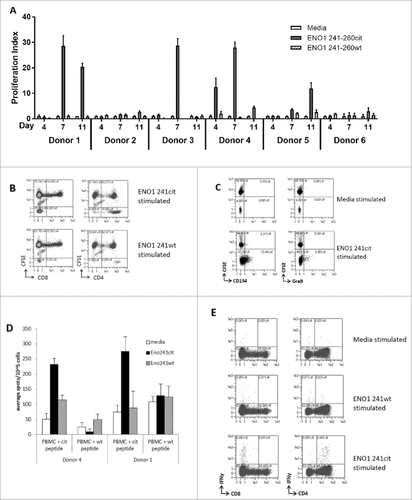
Figure 5. ENO1 241–260cit253 peptide induces responses in human PBMCs. PBMCs were isolated from 6 healthy donors and cultured with media, human ENO1 241–260cit253 or ENO1 241–260 wt peptide. Thymidine assays were performed to determine proliferation after 4, 7 and 11 days (A). HLA typing was performed on each donor (). PBMCs from donor 4 were labelled with CFSE prior to stimulation with peptides. The CD4 and CD8 populations within the CFSE labelled cell population was assessed by flow cytometry at day 10 (B). CD25-depleted CFSE labelled PBMCs were stimulated with media or ENO1 241–260cit253 peptide and analyzed by Flow cytometry on day 10. CD4+ gated samples are shown with staining for activation marker CD134 and cytokine Granzyme B (C). PBMCs from donor 1 and 4 were cultured for 13 days in the presence of ENO1 241–260cit253 or ENO1 241–260 wt peptide. PBMCs were then restimulated overnight and IFNγ responses were assessed by ELISpot (D). Flow cytometry staining at day 13 showed IFNγ staining in combination with CD4 and CD8 populations after restimulation with media, ENO1 241–260cit253 or ENO1 241–260 wt (E).