Figures & data
Figure 1. Clinical trial scheme and protocol. (A) Flow diagram illustrating the enrollment and assignment protocols and the analyses performed in the patients in this trial. In all, 37 eligible patients were allocated to receive the intervention treatment. However, 6 patients were lost during the treatment or follow-up period, and 31 patients were finally included for the clinical evaluation. (B) Overview of the clinical trial scheduling. Enrolled patients stopped cancer therapies so that they could generate activated DC-CIK Cells. Two weeks are required to manufacture DC-CIK cells. Anti-PD-1 antibodies were added, and the cells were then incubated for 30–40 min before the activated DC-CIK cells were infused. The patients received at least 8 cycles of infusions within 11 weeks. The response was assessed at 12, 24 and 36 weeks and quarterly thereafter. For eligible patients, maintenance infusions were given starting at 13 weeks.
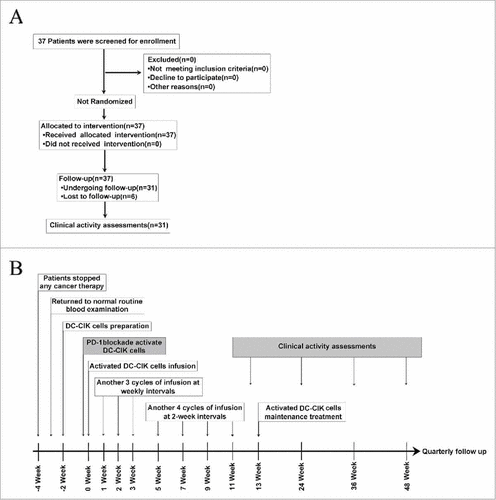
Table 1. Patient Characteristics.
Figure 2. Phenotype of DC-CIK cells at the time of infusion. The cells were predominantly CD3+ and CD8+T cells. A substantial proportion of PD-1+ cells (a median of 20.5%) were also detected in the final product.
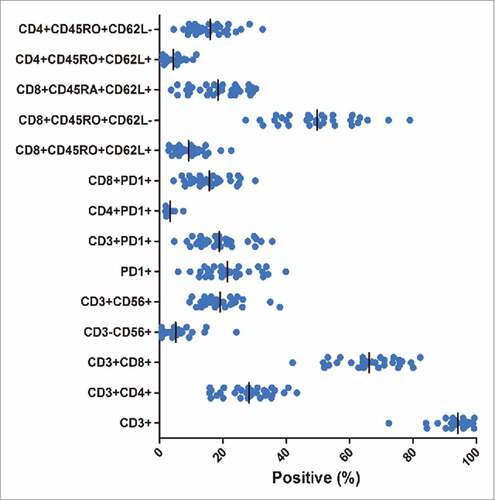
Table 2. Treatment-Related Adverse Events in patients in response to therapy (n = 31).
Figure 3. Clinical outcomes in 31 patients who were treated with PD-1 blockade-activated DC-CIK cells. (A) Spider plots showing the time and duration of clinical responses and changes in the tumor burden from baseline in patients who received pembrolizumab-activated autologous DC-CIK cell infusions. The thresholds for an objective response (-30%) and progressive disease (+20%) are marked by horizontal dashed lines, respectively, according to RECIST (version 1.1). (B) A waterfall plot of all patients showing the maximum reduction achieved in the target lesions from baseline as the sum of the longest diameter; +20% and −30% are marked by horizontal dashed lines. (C) Overall survival and progression-free survival were estimated using the Kaplan-Meier method for the 31 included patients. Censoring events are indicated by vertical tick marks.
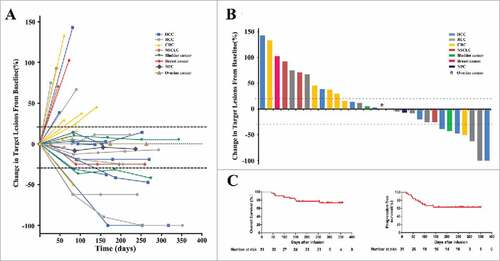
Table 3. Clinical effect of PD-1 blockade activated DC-CIK cells treatment.
Figure 4. Activity levels of PD-1 blockade-activated DC-CIK cells in three patients with advanced hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC). (A) A 61-year-old male with recurrent HCC experienced a complete response (CR) after receiving 14 cycles of pembrolizumab-activated autologous DC-CIK cell infusion. Although multiple intrahepatic metastases were observed on a baseline MRI (left), these lesions were no longer apparent at 24 weeks after the start of treatment (middle). The arrows indicate the regression of the lesions. The expression of the tumor marker alpha fetoprotein (AFP) had also decreased to a normal range after treatment (right). (B) A 34-year-old male with advance RCC achieved a CR after he was treated with pembrolizumab-activated autologous DC-CIK cells. Chest CT scans showing extensive bilateral pulmonary metastasis (arrow and circles) at baseline (left). This was followed by complete regression at 36 weeks after the start of the treatment (right). (C) Partial regression of metastatic RCC in a 31-year-old female within 12 weeks of the initiation of activated autologous DC-CIK cell infusions. The arrows show regression of ascites, and the yellow circle indicates regression of the lymph node metastases.
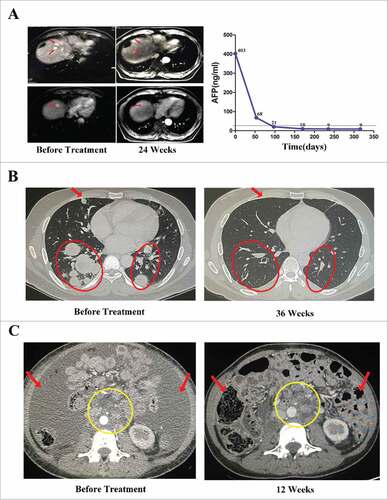
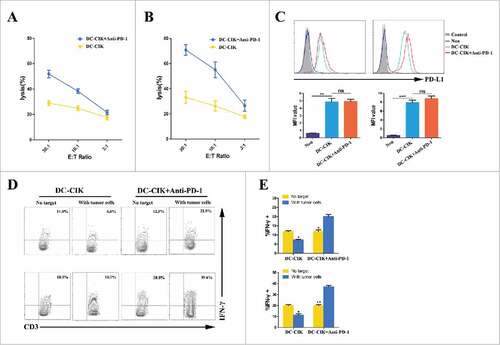
Figure 5. Cytotoxicity of DC-CIK cells that were derived from patients with advanced RCC. (A and B) The cytolytic activity of non-activated DC-CIK cells or activated DC-CIK cells in response to their respective autologous tumor cells, which were obtained from two RCC patients (patient 5942 (A) and patient 3435 (B)). E:T Ratio, effector cell to target cell ratio. (C) Changes in PD-L1 expression on tumor cells were analyzed using flow cytometry in the presence of non-activated DC-CIK cells or activated DC-CIK cells, as indicated, in patient 5942 (left) and patient 3435 (right). The corresponding mean fluorescence intensity (MFI) of three experiments is shown below the flow cytometry histogram plot. (D) Flow cytometric analysis of the expression of IFN-γ in non-activated DC-CIK cells or activated DC-CIK cells after the cells were co-cultured in medium or their respective autologous tumor cells. The samples were gated using CD3+ cells. Upper panels: patient 5942, lower panels: patient 3435. (E) The percentage of IFN-γ positive cells in three experiments is shown as a bar graph for patient 5942 (upper) and patient 3435 (lower). The data are presented as the mean ± SD. (#) P<0.05. (##) P<0.01, (###) P<0.001. ns, no significance.