Figures & data
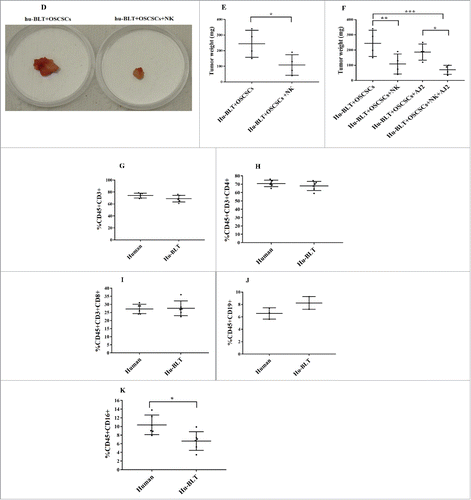
Figure 1. Single injection of super-charged NK cells with/without feeding AJ2 inhibited tumor growth in hu-BLT mice. Hu-BLT mice were generated as described in Materials and Methods, and shown in figure (A). Hu-BLT and NSG mice were implanted orthotopically with 1 × 106 human OSCSCs into the floor of the mouth, and after 7–10 days a group of hu-BLT mice were injected with 1.5 × 106 super-charged NK cells through tail vein, and mice were monitored for disease progression. Another group of hu-BLT mice were fed with AJ2 probiotic bacteria 5 billion/day every 48 hours 2 weeks prior to the implantation of OSCSCs and after implantation of the tumors in the presence and absence of NK injection until the experiments were terminated (B). Weight loss was monitored by weighing the mice on a weekly basis. One of 3 representative experiments is shown in this figure (C). Upon termination of the experiment, mice were sacrificed, and the pictures of tumors were taken after resection (D), and weighed (n = 4) (E). Mice were implanted with human OSCSCs and injected with NK cells and fed with AJ2, as shown in , and the tumors were resected and weighed post mortem (n = 4) (F). PBMCs were isolated from hu-BLT mice and humans and surface expression of human CD3 (n = 5) (G), CD4 (n = 5) (H), CD8 (n = 5) (I), CD19 (n = 3) (J) and CD16 (n = 5) (K) were determined within CD45+ immune cells using antibody staining followed by flow cytometric analysis.
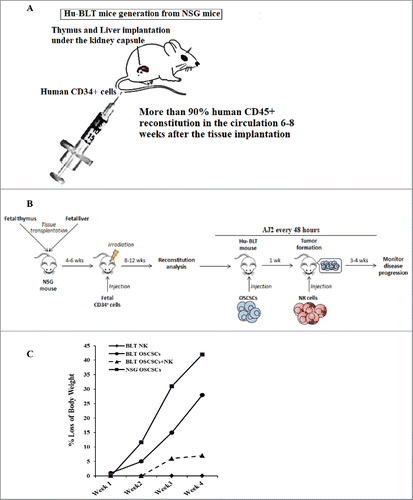
Figure 2. Injection of super-charged NK cells with/without feeding AJ2 restored and increased IFN-γ secretion and cytotoxic function of NK-cells in blood, spleen, BM, enriched-NK cells, and purified CD3+T cells in tumor-bearing hu-BLT mice. Hu-BLT mice were implanted with human OSCSCs and injected with NK cells, and fed AJ2 as described in , and a week after NK cell injection mice were injected with anti-PD1 (50 µg/mice) via tail-vein injection. Following sacrifice, spleen (n = 5) (A), BM (n = 5) (B) and peripheral blood (n = 5) (C) were collected, single cell suspensions were prepared from each tissue and (1 × 106 cells/ml for spleen and BM and 0.7 × 106 cells/ml for PBMCs) were treated with IL-2 (1000 U/ml) for 7 days. NK enriched cells were isolated from splenocytes, and (1 × 106 cells/ml) were treated with IL-2 (1000 U/ml) for 7 days (n = 3) (D). Cytotoxicity assays were performed using standard 4-hour 51Cr release assay against OSCSCs, and the LU 30/106 cells were determined using inverse number of cells required to lyse 30% of OSCSCs x100. Splenocytes (n = 5) (E), BM cells (n = 5) (F), PBMCs (n = 5) (G) at (1 × 106 cells/ml for spleen and BM and 0.7 × 106 cells/ml for PBMCs) were each treated with IL-2 (1000 U/ml), and positively selected CD3+T cells (n = 4) from the splenocytes at 1 × 106 cells/ml were treated with IL-2 (100 U/ml) (H), T cell depleted splenocytes were cultured at 1×106 cells/ml and treated with IL-2 (1000 U/ml) for 7 days, after which the supernatants were harvested and the levels of IFN-γ were determined using specific ELISAs (I). Fold changes in IFN-γ secretion in each tissue from each group of mice were determined over those obtained from mice injected with OSCSCs alone (E-H).
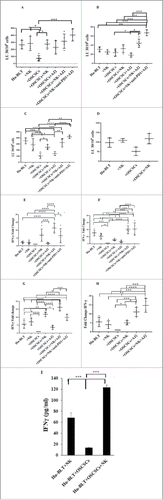
Figure 3. Single injection of super-charged NK cells with/without AJ2 feeding increased numbers of CD8+T cells in hu-BLT mice. Hu-BLT mice were implanted with OSCSCs and injected with NK cells, and fed with AJ2, as described in , and the percentages of human CD8+ T cells within BM cells (n = 3) (A) and splenocytes (n = 3) (B) were determined using antibody staining followed by flow cytometric analysis.
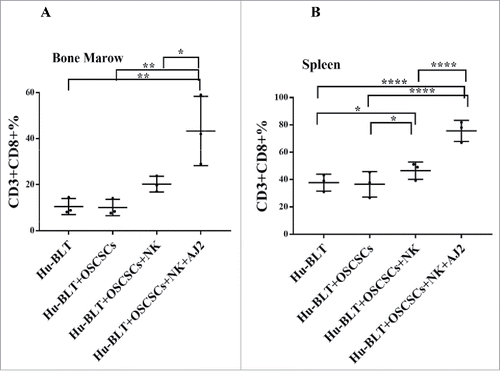
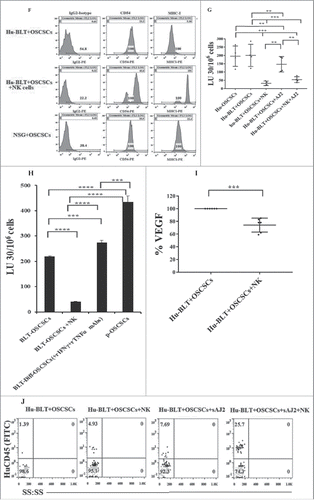
Figure 4. Single injection of super-charged NK cells with/without AJ2 feeding in hu-BLT mice mediated in vivo tumor differentiation, increased IFN-γ secretion and mobilized increased numbers of human immune cells to the tumors, and resulted in decreased ex-vivo tumor growth. Hu-BLT and NSG mice were implanted with OSCSCs and injected with NK cells, as described in . Following sacrifice, oral tumors were harvested, and single cell suspensions were prepared and the same numbers of cells (total of 3 × 106 cells at 1 × 106 cells/ml) from each group were cultured at day 0. On day 10 supernatants were removed and attached tumor cells were counted, and for subsequent cultures the numbers in each group were adjusted to those obtained from NK injected mice since they expanded the least numbers of tumors. On days 10, 14, 19 and 20 the total numbers of ex-vivo expanding tumor cells were determined in each group. One of several representative experiments is shown in this figure (A). Hu-BLT mice were implanted with OSCSCs or in vitro NK-differentiated-OSCSCs (diff-OSCSCs), or NK-differentiated-OSCSCs treated with antibodies against IFN-γ and TNF-α to block differentiation, followed by NK injection in hu-BLT mice as described in . Following sacrifice, oral tumors were dissociated, and single cells were prepared and cultured at (total of 3 × 106 cells at 1 × 106 cells/ml), and the numbers of expanding tumor cells were determined as described in . One of several representative experiments is shown in this figure (B). Hu-BLT mice were implanted with OSCSCs or diff-OSCSCs, or diff-OSCSCs treated with antibodies against IFN-γ and TNF-α, and injected with NK cells and/or fed with AJ2 as described in . Following sacrifice, oral tumors were harvested and cultured and the numbers of expanding tumor cells were determined as described in (n = 7) (C). Hu-BLT and NSG mice were implanted with OSCSCs, followed by NK injection in hu-BLT mice as described in . Oral tumors were harvested, and single cell suspensions were prepared. The percentages of infiltrating hu-CD45+ immune cells within the non-attached cells at day 12 of culture were determined using antibody staining followed by flow cytometric analysis. One of three representative figures is shown in this figure (D). Oral tumors from hu-BLT and NSG mice were cultured as described in and treated with IL-2 (1000 U/ml), and their supernatants were harvested on days shown in the figure and the levels of IFN-γ were determined using ELISA. One of several representative figures is shown in this figure (E). Expression of human CD54 and MHC-I were assessed on day 10 of oral tumor cultures from hu-BLT and NSG mice using flow cytometric analysis after staining with their respectiv antibodies. One of several representative experiments is shown in this figure (F). Purified NK cells (1 × 106 cells/ml) from the peripheral blood of the healthy human donor were left untreated or treated with IL-2 (1000 U/ml) for 18 hours before they were added to 51Cr labeled OSCSCs cultured from the resected tumors of different experimental groups of hu-BLT mice, and compared it to the cultures of OSCSCs maintained in the lab at various effector to target ratios. NK cell-mediated cytotoxicity was determined using a standard 4-hour 51Cr release assay. LU30/106 cells were determined as described in the Materials and Methods (n = 4) (G and H). Oral tumor cells from hu-BLT mice as described in were treated with IL-2 (1000 U/ml), and supernatants were harvested after 3 and 7 days and the levels of VEGF secretion were determined using specific ELISAs. The decrease in VEGF secretion by tumors obtained from NK-injected animals (n = 6) were calculated based on the amounts obtained from OSCSCs alone injected mice (I). Infiltrating percentages of hu-CD45+ immune cells within the oral tumors dissociated from different experimental groups of hu-BLT mice as described in Fig. S2C were determined using flow cytometric analysis after staining with antibody. One of several representative experiments is shown in this figure (J).
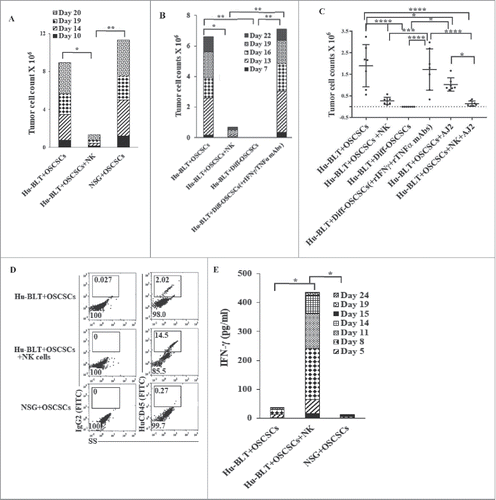
Figure 5. Single injection of super-charged NK cells with/without AJ2 feeding in tumor bearing mice restored and increased cytokine, chemokine and growth factor secretions within serum obtained from peripheral blood of hu-BLT mice. Serum from peripheral blood was obtained as described in the Materials and Methods section and multiplex arrays were performed to determine secretion of IFN-γ, one of the four representative figure is shown here (A). Fold changes of IFN-γ were determined based on the values obtained from control hu-BLT mice (n = 5) (B). Multiplex arrays were used to determine cytokines, chemokines, and growth factor secretion in sera obtained from the peripheral blood (C).
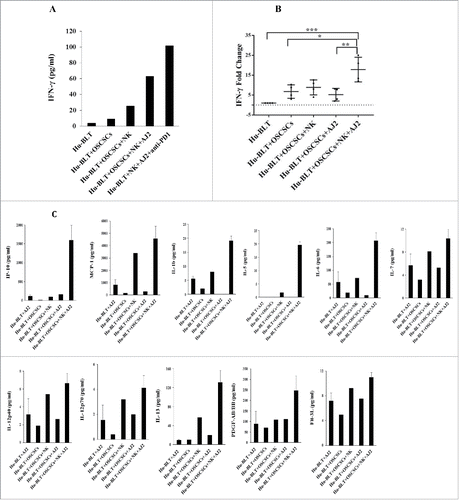
Figure 6. CDDP or Paclitaxel with and without NAC induce significant cell death in OSCSCs differentiated with NK-supernatants and not in poorly-differentiated tumors. Highly purified NK cells were treated with the combination of IL-2 (1000 U/ml) and anti-CD16mAb (3μg/ml) for 18 hours, after which the NK supernatants were added to OSCSCs in the presence of anti-TNF-α (1:100) and anti-IFN-γ (1:100) for a period of 5 days. Thereafter, OSCSCs were detached and treated with/without Cisplatin for 18–24 hours. The viability of OSCSCs was then determined using PI staining and flow cytometric analysis. One of 3 representative experiments is shown in this figure (A). OSCSCs were treated with the supernatants from NK cells as described in . Afterwards, tumors were detached and treated with/without NAC (20 nM) for 24 hours, followed by treatment with Paclitaxel for 18–24 hours. OSCSCs viability was determined by PI staining and flow cytometric analysis. One of 3 representative experiments is shown in this figure (B).
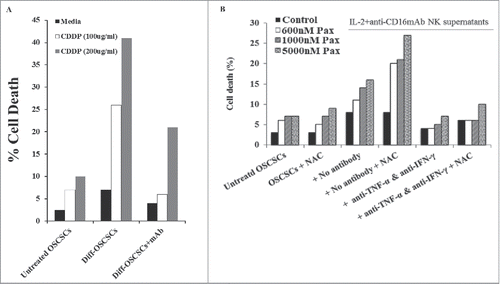
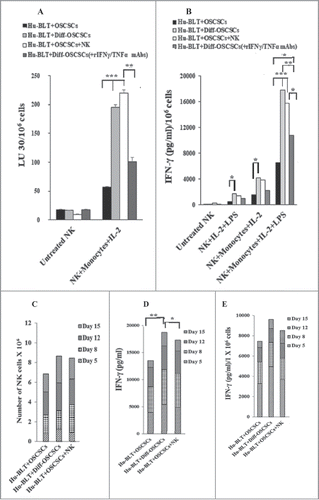
Figure 7. Monocytes or osteoclasts from tumor-bearing mice injected with super-charged NK cells or implanted with only NK-differentiated OSCSC-tumors induced significantly more IFN-γ from autologous or allogeneic NK-tumor co-cultures when compared to those of tumor-alone implanted mice with NK cells. Hu-BLT mice were implanted with OSCSCs and injected with NK cells, and fed AJ2 as described in . After sacrifice, NK cells from splenocytes and monocytes from BM cells were isolated, as described in Material and Methods section. Autologous NK cells were left untreated or treated with IL-2 (1000 U/ml) in combination with monocytes (NK:monocytes, 2:1) and at day 7 after the co-culture, NK cells were used as effector cells in a standard 4-hour 51Chromium release assay against OSCSCs. The LU 30/106 cells were determined using inverse number of NK cells required to lyse 30% of the target cells X100 (A). Autologous NK cells were left untreated or treated with IL-2 (1000 U/ml) or with the combination of IL-2 (1000 U/ml) and LPS (100 ng/ml) in the absence and presence of monocytes (NK:monocytes, 2:1) for 7 days, after which the supernatants were harvested and IFN-γ secretion were determined using single ELISA (B). OCs were generated from purified hu-BLT monocytes, as described in Material and Methods section. Purified allogeneic NK cells from healthy human donors were pre-treated with IL-2 (1000 U/ml) and anti-CD16mAb (3 µg/ml) for 18 hours and then cultured with hu-BLT-OCs in the presence of sAJ2 (NK:OCs:sAJ2, 2:1:4). After culture, numbers of NK cells in the culture were counted on day 5, 8, 12 and 15 using microscopy (C). The supernatants were harvested from cultures on days 5, 8, 12 and 15 and the IFN-γ secretion was determined using single ELISA (D). The levels of IFN-γ obtained from ELISA were determined in 1 × 106 cells using cell counts from (E).