Figures & data
Figure 1. DNA- and protein-based intradermal vaccination generates Trm precursors in blood and Trm cell responses in the skin. C57 BL/6 mice were intravenously transferred with OVA-specific CD45.1+ OTI CD8+ T cells and a day later, intradermally vaccinated with DNA-OVA or Protein-OVA. Control mice (CTRL) were vaccinated with empty plasmid (for DNA vaccination) or unvaccinated (for Protein vaccination). a, b Analysis of Teff responses in blood twelve days after vaccination by flow cytometry. (a) Representative dot-plot showing the expression of CD44 and CD45.1 in total CD8+ T cell population (left panel). Graphs with the percentage of CD44+ CD45.1+ OVA-specific Teff cells. (b) Representative dot-plot of KLRG1 and CD127 expression in CD45.1+ Teff cells (left panel). Representative histograms showing the expression of CXCR3, P-selectin ligand (PSL) and E-selectin ligand (ESL) in OVA-specific memory precursors (KLRG1low CD45.1+ Teff cells). c-e Analysis of memory responses in skin 4–5 weeks after vaccination by flow cytometry. (c) Representative dot-plots of total CD45+ live cells showing the presence of OVA-specific memory CD8+ T cells in vaccinated (V) and distant (D) skin. (d) Representative dot-plots and graphs showing OVA-specific Trm cells generated in vaccinated and distant skin after DNA-OVA (top) and Protein-OVA (bottom) vaccination. OVA-specific Trm cells were defined as CD3+CD8+CD45.1+CD103+CD69+ cells. (e) Representative histograms showing expression of CD44, PD-1 and CD127 analyzed in CD45.1+ OVA-specific Trm cells. (a, d) Pooled data of two independent experiments, n = 10 mice per group in a, and n = 7 mice per group in d. Bars are the mean ± SEM. ***p < 0.001; ****p < 0.0001 by Mann-Whitney unpaired t test.
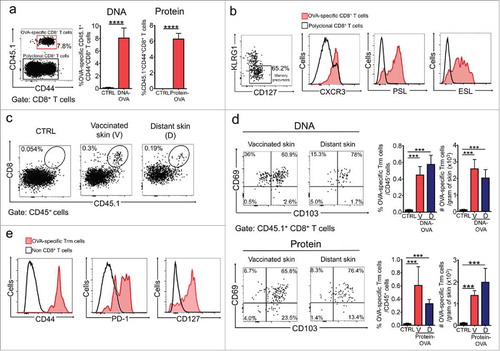
Figure 2. Vaccination-induced skin Trm cells are largely refractory to intravascular staining and antibody-dependent depletion. C57 BL/6 mice were intravenously transferred with OVA-specific CD45.1+ OTI CD8+ T cells, the day after were intradermally vaccinated with DNA-OVA or Protein-OVA. Trm cell and circulating memory responses were analyzed 4–5 weeks later by flow cytometry. a Intravascular staining was performed by injecting mice with anti CD8β antibody intraperitoneally before the analysis. Representative dot-plots and quantification of CD8β− and CD8β+ OVA-specific CD45.1+ OTI CD8+ T cells in skin and lungs after intravascular staining. b-d Mice were treated with isotype-matched control (αCTRL) or anti-CD8 (αCD8) antibodies to eliminate circulating CD8+ T cells. Vaccination-induced memory responses were analyzed one week after antibody-dependent depletion. Representative dot-plot and graphs showing CD45.1+ OTI memory CD8+ T cells cells in skin (b), spleen (c) and lungs (d). Pooled data of two independent experiments, n = 7 mice per group. Bars are the mean ± SEM. ***p < 0.001, ****p < 0.0001, ns = non-significant by Mann-Whitney unpaired t test.
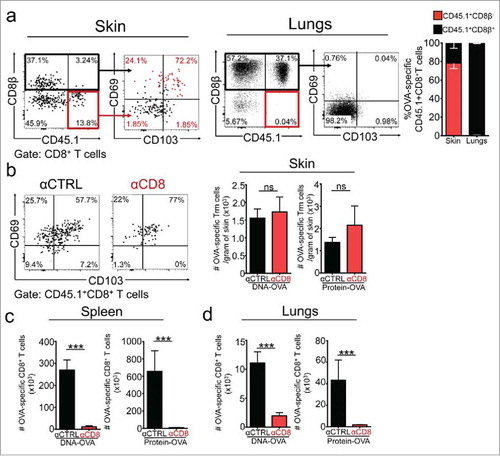
Figure 3. Vaccination-induced skin Trm cells mediate strong protection against cutaneous melanoma independently of circulating CD8+ T cells. C57 BL/6 mice were intravenously transferred with OVA-specific CD45.1+ OTI CD8+ T cells and the day later, were intradermally vaccinated with DNA-OVA or Protein-OVA. Control mice (CTRL) were vaccinated with empty plasmid (for DNA vaccination) or left unvaccinated (for protein vaccination). After 4–5 weeks, mice were depleted of circulating CD8+ T cells by administering anti-CD8 antibody and one week later, were intradermally challenged with melanoma cells. a-c Experimental scheme (a) and tumor growth curves of mice challenged with B16F10-OVA (b) and B16F10-OTI (c) close to the vaccinated site. (d) Tumor growth curves of mice challenged at distant skin sites with B16F10-OVA (left) and B16F10-OTI (right). Data is representative of two independent experiments, n = 5 and n = 4 for DNA and protein vaccines, respectively. Bars are the mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001 by Two-way ANOVA and Bonferroni post-hoc test.
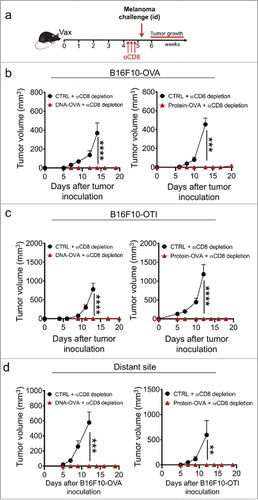
Figure 4. Skin Trm cells generated from the endogenous T cell repertoire protect against melanoma. C57 BL/6 mice were intradermally vaccinated with DNA-OVA. Control mice were vaccinated with empty pVAX plasmid (CTRL). a. Effector responses were analyzed in blood twelve days after vaccination by H-2Kb/OVA(257–264) multimer and CD44 stainings followed by flow cytometry. Representative dot-plots and graphs showing OVA-specific Teff cells in total CD8+ T cell population. b. The generation of OVA-specific skin Trm cell responses was evaluated 4–5 weeks after vaccination by H-2Kb/OVA(257–264) (KbOVA) multimer staining followed by flow cytometry. Representative dot-plots and quantification of OVA-specific (KbOVA+) memory CD8+ T cells in total CD45+ live cells present in vaccinated (V) and distant (D) skin. OVA-specific Trm cells were defined as CD3+CD8+KbOVA+CD103+CD69+ cells. c. Mice were depleted of circulating CD8+ T cells 4–5 weeks after vaccination and one week later, were intradermally challenged with B16F10-OVA cells near the vaccinated site (bottom left) or at a distant site (bottom right). Data is representative of two independent experiments, bars are the mean ± SEM, n = 5 mice per group. *p < 0.05, **p < 0.01 for (a, b) by Mann-Whitney unpaired t test; ***p < 0.001 and ****p < 0.0001 for (c) by Two-way ANOVA and Bonferroni post hoc test.
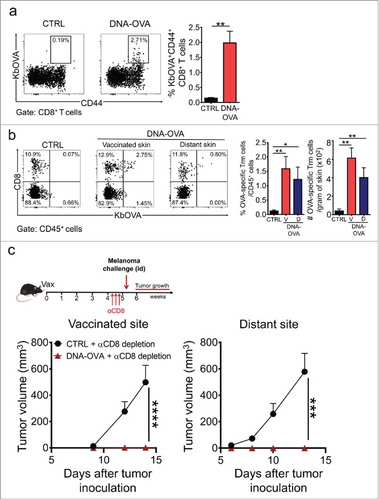
Figure 5. Route of vaccine administration determines the generation of Trm precursors and protective Trm cell responses against melanoma. C57 BL/6 mice were intravenously transferred with OVA-specific CD45.1+ OTI CD8+ T cells and a day later, were intradermally (ID) or intraperitoneally (IP) vaccinated with a Protein-OVA (αDEC-OVA plus polyI:C). a, b Analysis of Teff responses in blood by flow cytometry, twelve days after vaccination. (a) Graph showing quantification of OVA-specific CD45.1+ OTI CD8+ T cells. (b) Representative histograms showing expression of CXCR3, E-selectin ligand (ESL) and P-selectin ligand (PSL) in CD45.1+ OTI CD8+ T cells. c, d Analysis of memory responses in spleen (c) and skin (d) by flow cytometry, 4–5 weeks after vaccination. (c) Quantification of CD45.1+ OTI CD8+ T cells in spleen. (d) Representative dot-plot of CD8 and CD45.1 expression in CD45+ total live cells and quantification of OVA-specific Trm cells generated in skin after ID or IP vaccination. e Experimental scheme (top) and tumor growth curves (bottom) of mice treated with isotype-matched control (αCTRL, left) or anti-CD8 (αCD8, right) antibodies, and challenged with B16F10-OVA melanoma cells. (a, c, d) Pooled data of two independent experiments, n = 7 mice per group. (e) Data is representative of two independent experiments, n = 4 mice per group. Bars are the mean ± SEM. **p < 0.01, ns = non-significant for (a, c, d) by Mann-Whitney unpaired t test. ***p < 0.001 for (e) by Two-way ANOVA and Bonferroni post hoc test.
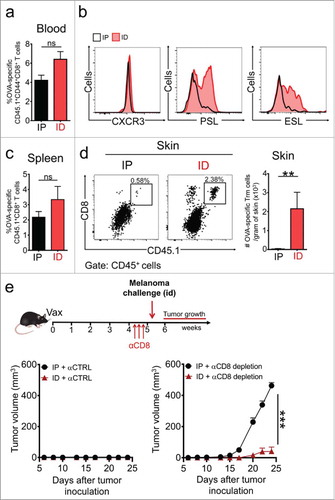
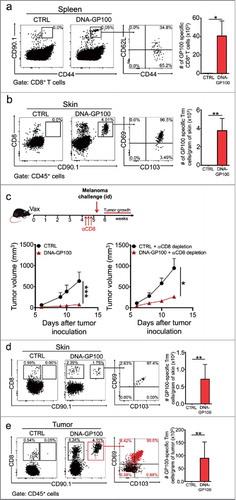
Figure 6. Vaccination with a melanoma-associated self-antigen generates specific Trm cell responses and suppresses tumor growth. C57 BL/6 mice were intravenously transferred with GP100-specific CD90.1+ pMEL-1 CD8+ T cells and the day afterwards, intradermally vaccinated with a DNA vaccine encoding the melanoma-associated antigen GP100 (DNA-GP100). Control mice were vaccinated with empty pcDNA plasmid (CTRL). a-b GP100-specific memory responses were analyzed 4–5 weeks after vaccination in spleen (a) and skin (b) by flow cytometry. Representative dot-plots of CD90.1+ GP100-specific memory CD8+ T cells in total CD8+ T cell (a) and CD45+ live (b) populations. Memory phenotype and total number quantifications are also shown for each case. c-e Mice were either non-depleted or depleted of circulating CD8+ T cells by injecting anti-CD8 antibody (αCD8) 4–5 weeks after vaccination. One week later, mice were intradermally challenged with B16F10 melanoma cells. (c) Experimental scheme and tumor growth curves are shown for non-depleted (bottom left) and CD8+ T cell-depleted (bottom right) mice. (d-e) CTRL and DNA-GP100 vaccinated mice depleted of circulating CD8+ T cells were sacrificed twelve-days after tumor challenge, and skin (d) and tumors (e) were analyzed by flow cytometry. Representative dot-plots of CD8 and CD90.1 expression in CD45+ live cells (left panels), and CD103 and CD69 expression in GP100-specific CD90.1+ CD8+ T cells (middle panel). In the case of tumor analysis, CD90.1+ (red dots) and CD90.1− (black dots) CD8+ T cells were overlaid. Percentages of CD90.1+ CD8+ T cells for each quadrant are indicated. Quantification of total GP100-specific Trm cells, defined as CD3+CD8+CD90.1+CD103+CD69+ T cells are shown for each case (right panels). (a, b, d, e) Pooled data of two independent experiments, bars are the mean ± SEM, n = 6 mice per group. (c) Data is representative of two independent experiments, n = 3 mice per group. Bars are the mean ± SEM. *p<0.05, **p<0.01 for (a, b, d, e) by Mann-Whitney unpaired t test. *p<0.05 and ***p<0.001 by Two-way ANOVA and Bonferroni post hoc test.