Figures & data
Figure 1. Schema of protocol therapy. Abbreviations: NK = Natural Killer; VINC = Vincristine; CY = Cyclophosphamide; TO = Topotecan.
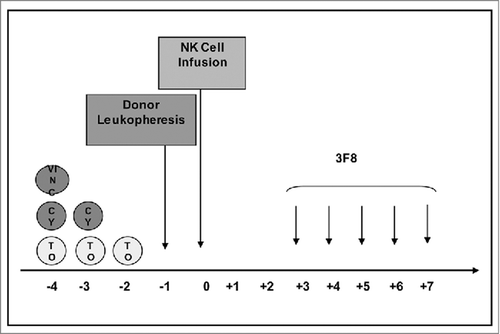
Table 1. Clinical features and results of genotyping on patients and donors.
Table 2. Planned and actual dosage of haploidentical NK cells administered.
Table 3. Numbers of patients experiencing toxicities.
Table 4. Responses.
Table 5. Relationships between response and dose and other factors.
Figure 2. Complete response to protocol therapy in a 6-year old male with chemorefractory high-risk stage 4 neuroblastoma after 2 cycles of therapy at NK-cell dose level 3. Pre-therapy 123I-MIBG scan showed uptake in multiple skeletal areas including pelvis, bilateral femora and spine. Post-therapy 123I-MIBG scan showed complete resolution of skeletal uptake. Complete response was also noted on bone marrow histology.
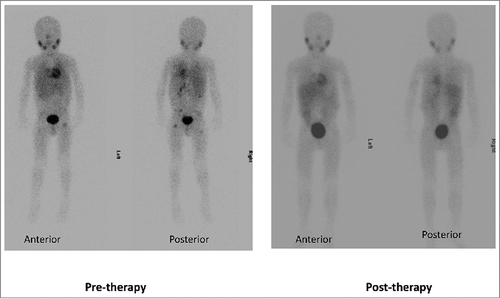
Table 6. Correlation of NK cytotoxicity and donor-recipient HLA/KIR phenotyping.
Figure 3. Donor and patient NK function. NK-cells were isolated from donors, the patient pre-treatment, and the patient post-treatment at indicated time points. NK response capacity was assessed by CD107a mobilization to (A) the NK-sensitive K562 cell line, or to (B) the neuroblastoma cell line LAN-1 in the presence of the m3F8 monoclonal antibody. 13 donors and 19 patients were assessed. Each bar represents the mean +/− SEM.
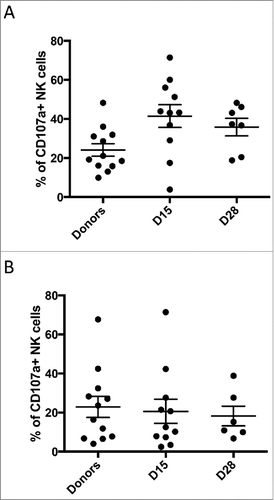
Figure 4. NKG2A expression among donors and patients. A high percentage of NKG2A expression was noted in the patient population pre- and post-infusion (A) compared to a healthy adult population (B).
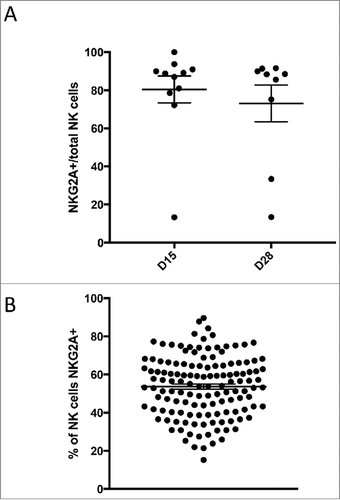
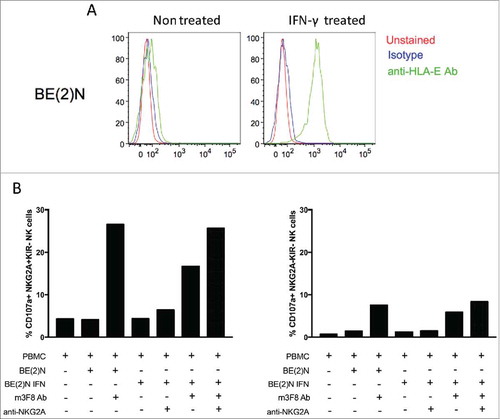
Figure 5. HLA-E expression on NB selectively inhibits NKG2A-expressing NK cells. (A) HLA-E is readily upregulated on the cell surface of the NB cell line BE(2)N upon IFN-γ treatment. (B) m3F8-induced CD107a response among NKG2A+KIR- NK cells (left panel) or NKG2A-KIR- NK cells (right panel) to the IFN-γ treated or untreated BE(2)N target cell in the presence or absence of the anti-NKG2A blocking antibody. Similar results were noted with SKNLH cell line (data not shown)