Figures & data
Table 1. Demographic characteristics of advanced melanoma and NSCLC cohorts (response data set).
Table 2. Clinical response evaluated among melanoma and NSCLC patients treated concomitantly with denosumab and immune checkpoint inhibitors.
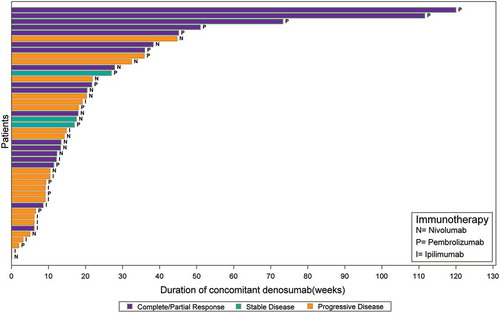
Figure 1. A Clinical response among advanced melanoma patients by duration of concomitant treatment with denosumab and immune checkpoint inhibitors (n = 44). B Clinical response among advanced NSCLC cohort by duration of concomitant treatment with denosumab and immune checkpoint inhibitors (n = 166).
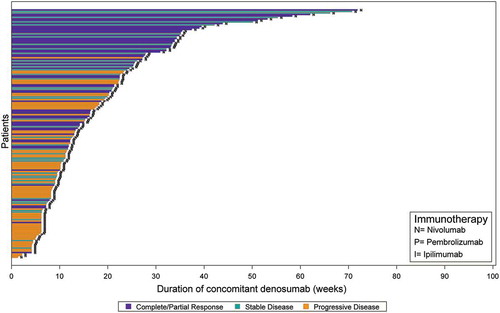
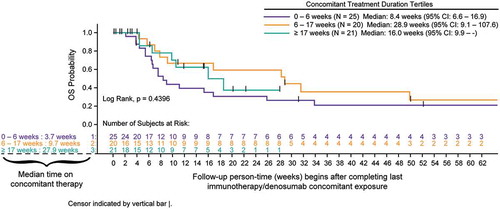
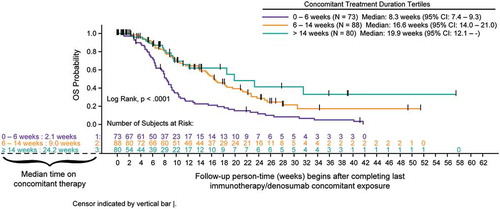
Figure 2. A Overall survival for advanced melanoma cohort measured after completion of concomitant treatment duration (tertiles) of denosumab and immune checkpoint inhibitors (full analysis set). B Overall survival for advanced NSCLC cohort measured after completion of concomitant treatment duration (tertiles) of denosumab and immune checkpoint inhibitors (full analysis set). C Overall survival for advanced melanoma and NSCLC cohorts measured after completion of combined by best response category achieved with concomitant denosumab and immune checkpoint inhibitors (best response set).
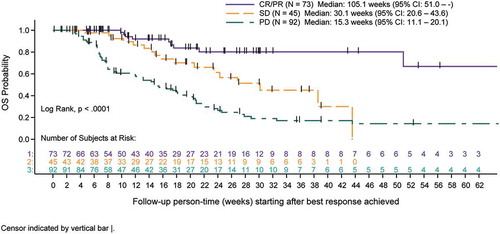
Figure 3. Optimal anti-tumor efficacy of anti-PD1 and anti-RANKL is affected by sequencing of antibody administration. Groups of C57Bl/6 wild type (WT) mice (n = 10/group) were injected s.c with 5 × 10Citation5 3LL lung carcinoma cells. For concurrent treatment groups (black symbols), mice were treated i.p. on days 8, 12, 16 and 20, relative to tumor inoculation (as indicated by arrows) with cIg (1–1, 100 µg), anti-PD1 (RMP1–14, 100 µg) and/or anti-RANKL (IK22/5, 100 µg) as indicated. For sequential treatment groups (colored symbols), where treatment order is indicated in figure legend, mice were treated i.p. on days 8 and 12 (first antibody) and days 16 and 20 (second antibody) respectively (relative to tumor inoculation) with cIg (1-1, 200 µg), anti-PD1 (RMP1-14, 200 µg) and/or anti-RANKL (IK22/5, 200 µg) as indicated. Mean ± SEM tumor size is shown for each treatment group. Statistical differences between groups at day 22 were determined by one-way ANOVA with Tukey’s post-test analysis, and key comparisons are shown (*p < 0.05, **p < 0.01, ****p < 0.0001). Two independent experiments have been pooled.
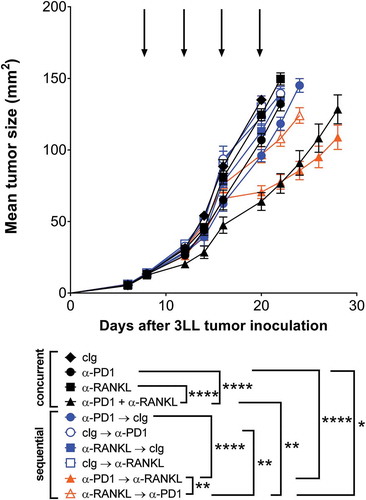
Table 3A. Comparison of objective response rates from anti-PD-1 pivotal clinical trials in NSCLC.
Table 3B. Comparison of anti-PD1 pivotal clinical trials in melanoma.