Figures & data
Figure 1. AZD1775 sensitizes antigen-positive M1mK2SIIN cells to perforin/granzyme B-dependent OT-I CTL killing.
A, pMOC1 or M1mK2SIIN cells with or without IFNγ (20 ng/mL for 24 hours) were evaluated for mKate2 fluorescence and the presentation of SIINFEKL presentation via H-2Kb via flow cytometry.B, growth control or killing of M1mK2SIIN cells with OT-I CTL (added at the indicated vertical line) at varying E:T ratios. 10:1 represents the minimum E:T ratio that does not allow M1mK2SIIN cell recovery over time. Representative impedance plot on left and quantification of loss of cell index at 48 hours (after the addition of CTL) on right. OT-I CTL control of pMOC1 cells was quantified at 48 hours as a negative control.C, M1mK2SIIN cells were plated at time 0 in the presence of AZD1775 (250 nM) or DMSO (volume equivalent, control) and CTL were added at the indicated vertical line, with quantified loss of cell index at 12 and 48 hours after the addition of CTL.D, SIINFEKL presentation via H-2Kb was quantified on M1mK2SIIN cells treated with AZD1775 (250 nM) or DMSO alone via flow cytometry. Pre-treatment with IFNγ (20 ng/mL for 24 hours) was used as a positive control.E, either M1mK2SIIN tumor cells or OT-I CTL alone were exposed to AZD1775 (250 nM) or DMSO alone and CTL growth control or killing of M1mK2SIIN cells was assayed, with quantified loss of cell index at 12 and 48 hours after the addition of CTL. For tumor cells exposure alone, M1mK2SIIN cells were plated in AZD1775 and time 0 and washed three times before the addition of CTL. For OT-I CTL exposure alone, AZD1775 was added to CTL culture for 24 hours, and CTL were washed three times before addition to the impedance assay.F, OT-I CTL killing of M1mK2SIIN cells treated with AZD1775 (250 nM) or DMSO alone was assayed in the presence or absence of the perforin inhibitor concanamycin A (OT-1 CTL treated with 100 nM for 4 hours prior to addition to impedance assay), with quantification 18 hours after the addition of CTL.**, p < 0.01, ***, p < 0.001; n/s, non-significant. Representative data (mean±SD) from one of three independent assays for each experiment, each performed in technical triplicate.
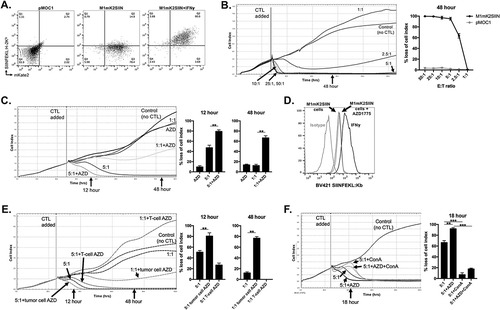
Figure 2. Mixing M1mK2SIIN and pMOC1 cells allows in vitro modeling of antigen escape.
A, SIINFEKL positive (M1mK2SIIN) and SIINFEKL negative (pMOC1) cells were mixed at the ratios indicated on the right side of the plot and assayed for growth control following the addition of 10:1 CTL at the indicated vertical line, quantified at 90 hours after the addition of CTL. *, p < 0.05 in relation to the previous dilution. Representative data from one of three independent assays.B, OT-I CTL (5:1 E:T) growth control of a mixture of 15% SIINFEKL negative (pMOC1) cells was assayed, with addition of fresh OT-I CTL (5:1 E:T, dashed vertical line) after tumor cell growth rebound. Control tumor cells (no CTL exposure) and tumor cells that had escaped 5:1 CTL control were assessed for mKate2 positivity via flow cytometry. Representative data from one of two independent assays.
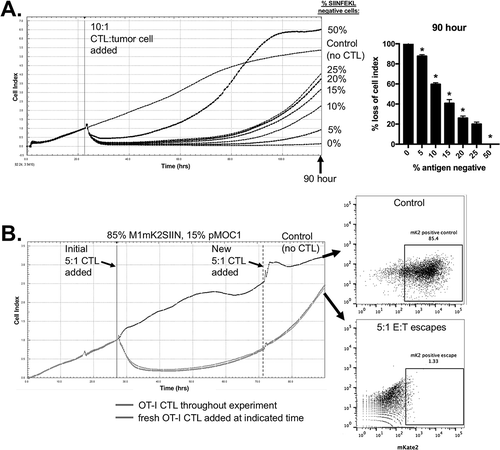
Figure 3. AZD1775 sensitizes SIINFEKL negative cells to transmembrane TNFα-dependent control by antigen specific CTL.
A, OT-I CTL (10:1 E:T) control of 15% SIINFEKL negative pMOC1 tumor cells in the presence of AZD1775 (250 nM) or DMSO alone was assayed. Monoclonal antibodies targeting TNFα (clone XT3.11), FasL (MFL3) or TRAIL (N2B2; all 2 μg/mL) were added with CTLs to some wells (CTL and mAbs mixed before addition to target cells), with quantification 60 hours after the addition of CTL.B, OT-I CTL (10:1 E:T) control of 15% SIINFEKL negative tumor cells in the presence of AZD1775 (250 nM) or DMSO alone was assayed. In some wells, SIINFEKL negative pMOC1 cells were replaced with TNFR1 knockout pMOC1 cells, with quantification 60 hours after the addition of CTL.C, OT-I CTL (10:1 E:T) control of 15% SIINFEKL negative pMOC1 tumor cells in the presence of AZD1775 (250 nM) or DMSO alone was assayed. In some wells, TAPI-0 (10 μM) was added with or without TNFα mAb (2 μg/mL) with CTLs. When used, CTLs were exposed to TAPI-0 for 2 hours prior to being added to target cells. Quantification of loss of cell index performed 72 hours after the addition of CTLs.D, OT-I CTL (10:1 E:T) growth control or killing of 15% SIINFEKL negative pMOC1 cells in the presence of AZD1775 (250 nM) or DMSO alone was assayed via impedance analysis in the presence or absence of ZVAD (20 μM) or necrostatin (20 μM) alone or in combination, with quantification 72 hour after the addition of CTL.E, OT-I CTL (10:1 E:T) control of 15% SIINFEKL negative tumor cells in the presence of AZD1775 (250 nM) or DMSO alone was assayed. In some wells, SIINFEKL negative pMOC1 cells were replaced with β2M knockout pMOC1 cells, with quantification 72 hours after the addition of CTL.F, SIINFEKL positive M1mK2SIIN (85%) and antigen-negative β2M knockout pMOC1 cells (15%) were co-incubated for 24 hours, stimulated together with 10 ng/mL IFNγ for an additional 24 hours, and assessed for SIINFEKL presentation on H-2Kb via flow cytometry.*, p < 0.05, **, p < 0.01; ***, p < 0.001; n/s, non-significant. Representative data from one of three independent assays for each experiment.
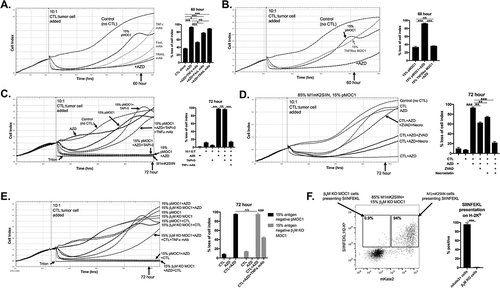
Figure 4. Early granzyme B- and late TNFα-induced cell cycle pause at G2/M is reversed in the presence of AZD1775 leading to DNA damage and subG1 accumulation.
A, pMOC1 cells were treated with AZD1775 (250 nM for 24 hours) or DMSO control followed by streptolysin O (40 ng/mL)/granzyme B (1500 ng/mL) or TNFα (20 ng/mL) for 2 or 8 hours before 4 marker (DNA content, EdU, p-HH3S10 and p-γH2AXS139) flow cytometry analysis. Representative flow cytometry dot plots and p-HH3 histogram from G2/M gated cells shown.B, quantification of accumulation of cells within specific phases of the cell cycle following treatments as in A. Pooled data from three independent experiments, each performed in technical triplicate.C, quantification of p-γH2AXS139 mean fluorescence intensity (MFI) by cell cycle phase following treatments as in A. Pooled data from three independent experiments, each performed in technical triplicate.D, representative half-offset flow cytometry histograms demonstrating p-γH2AXS139 staining intensity during M-phase from selected conditions. Specific MFI for each condition listed adjacent to each histogram.E, pMOC1 cells irradiated to 8Gy were used as a positive control for cell cycle analysis. Representative dot plot shown on the left, with overlaid histogram demonstrating p-γH2AXS139 staining intensity in each phase of the cell cycle on the right.F, pMOC1 cells were treated as in A and expression and phosphorylation of select proteins was assessed via western blot analysis at the indicated time points.*, p < 0.05, **, p < 0.01; ***, p < 0.001
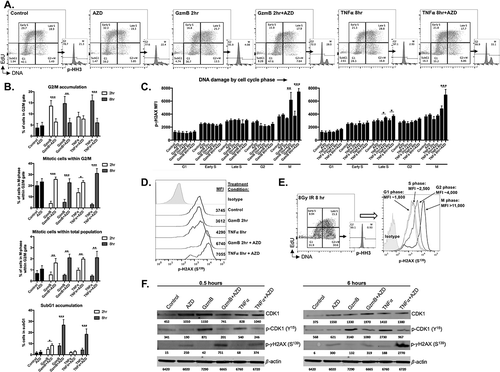
Figure 5. AZD1775-induced sensitization of SIINFEKL negative cells to CTL control is reproduced in an array of cancer models.
The mKate2-SIINFEKL retroviral construct was stably transduced into (A) B16 melanoma, (B) MOC2 oral cavity squamous cell carcinoma and (C) MC38 colon adenocarcinoma cell lines. Antigen positive (mK2SIIN positive variants) cells and mixtures of 15% SIINFEKL negative (parental variant) cells for each cell line, plated at time 0 in AZD1775 (250 nM) or DMSO alone (control), were assayed for growth control following the addition of CTL (at different E:T ratios) at the indicated vertical line. Quantification for each cell line at the time point indicated is shown to the right.*, p < 0.05, **, p < 0.01; ***, p < 0.001. Representative data from one of three independent assays for each experiment.
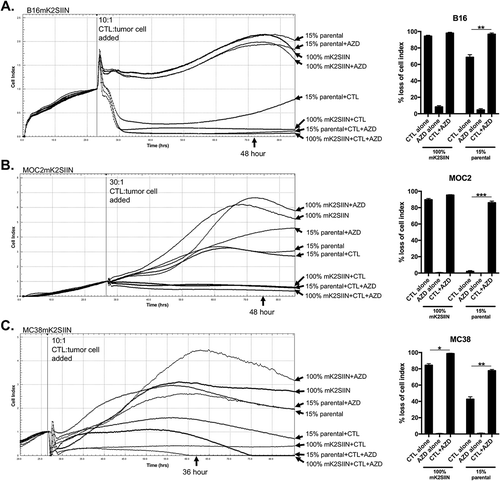
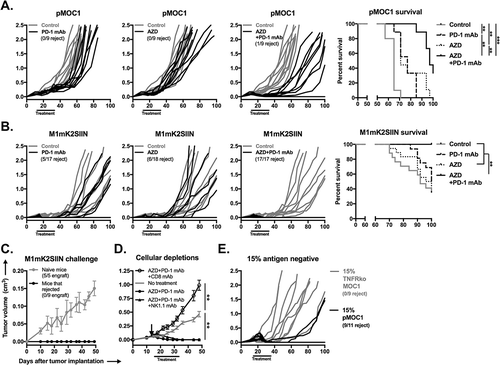
Figure 6. M1mK2SIIN tumors engraft but escape anti-tumor immunity despite persistent antigen expression.
A, M1mK2SIIN or pMOC1 cells (5x106) were implanted subcutaneously in the flanks of WT B6 mice, and tumors were allowed to engraft and grow; plotted are individual tumor growth curves.B, established M1mK2SIIN or pMOC1 tumors were harvested, digested into a single cell suspension, and assayed for mKate2 positivity via flow cytometry. Dead cells were excluded via sytox blue staining.C, total infiltrating CD45.2+CD3+CD8+ TIL, PD-1 expression on CD8+ TIL, SIINFEKL:H-2Kb tetramer positivity and PD-L1 expression on CD45.2−CD31− cells was assessed in digested M1mK2SIIN and pMOC1 tumors via flow cytometry; total tumor IFNγ expression was determined via qRT-PCR of whole tumor lysate. Data pooled from two independent experiments.
Figure 7. Combination AZD1775 and PD-1 mAb treatment induces consistent CD8+ cell-dependent rejection of established SIINFEKL positive and mixed SIINFEKL negative tumors.
A, established pMOC1 tumors (5x106 cells/mouse) were treated with PD-1 mAb (clone RPM1-14, 200 μg/injection IP twice weekly for 3 weeks) or AZD1775 (60mg/kg OG 5 days on, 2 days off for 3 weeks) alone or in combination beginning at day 14; individual growth curves are shown (n = 9–10 mice/group). Timing of treatments indicated by black bar along x-axis. Control mice were treated with IP injection of rat IgG2a isotype control antibody and/or oral gavage of 0.5% methylcellulose carrier. Number of mice that rejected established tumors indicated below legends. Survival analysis displayed on right. Kaplan-Meier curves were compared using the log-rank/Mantel Cox test.B, established M1mK2SIIN tumors (5x106 cells/mouse) were treated as in A (n = 8–9 mice/group, pooled data from two independent experiments). Survival analysis displayed on right.C, mice that rejected established M1mK2SIIN tumors with combination AZD1775 and PD-1 mAb treatment or naïve WT B6 mice were challenged with M1mK2SIIN cells (3x106) injected subcutaneously into the contralateral flank and followed for tumor engraftment and growth.D, WT B6 mice were engrafted with M1mK2SIIN tumors and treated with combination AZD1775 and PD-1 mAb as in A, but with the addition of CD8+ cell depletion (clone YTS169.4, 200 μg IP twice weekly) or NK1.1+ cell depletion (clone PK136, 200 μg IP twice weekly) beginning one day prior to treatment (day 13, black arrow) and followed for tumor growth (n = 5–7 mice/group). Tumor volume at day 48 was compared to assess statistical significance via ANOVA.E, WT B6 mice were engrafted with a mixture of 85% antigen positive M1mK2SIIN cells and 15% antigen negative either pMOC1 or TNFR1 ko pMOC1 cells (5x106 cells/mouse total, n = 9–11 mice/group). Mice were treated with combination AZD1775 and PD-1 mAb as in A, and followed for tumor growth.**, p < 0.01; ***, p < 0.001.