Figures & data
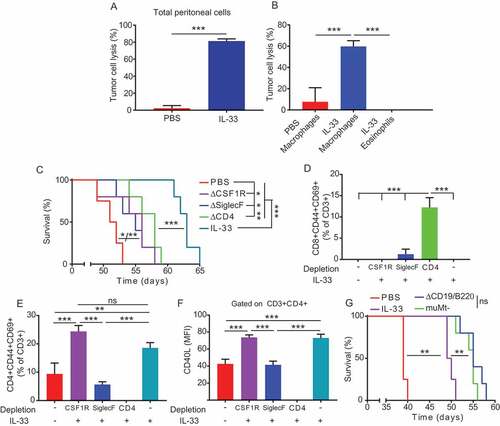
Figure 1. IL-33 delays ovarian cancer tumor progression.
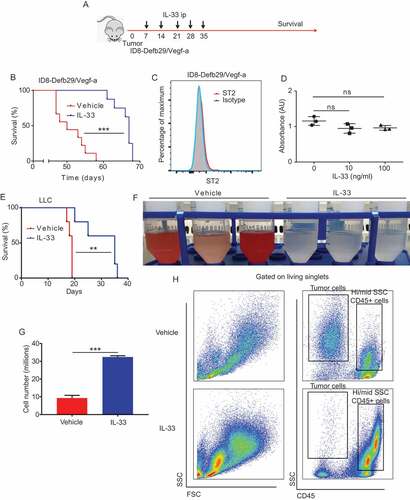
Figure 2. IL-33 promotes an allergic like infiltration of the peritoneal cavity.
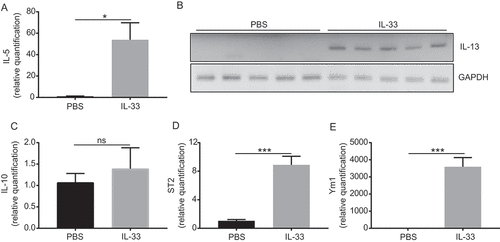
Figure 3. IL-33 promotes recruitment and activation of peritoneal CD4 T cells.
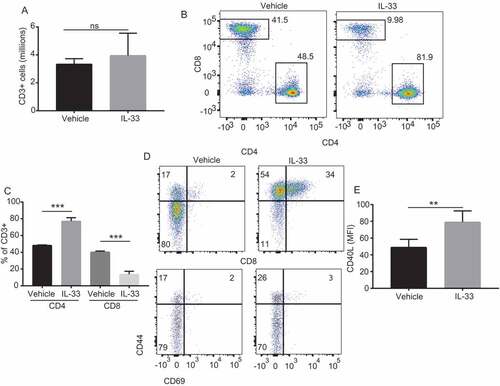
Figure 4. IL-33 promotes peritoneal CD4 T-cell and B cell activation and eosinophil recruitment.
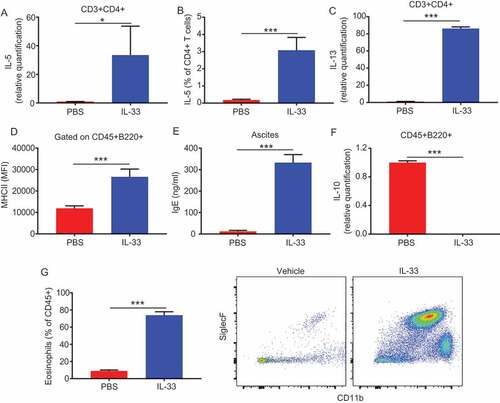
Figure 5. IL-33 promotes activation of peritoneal macrophages.
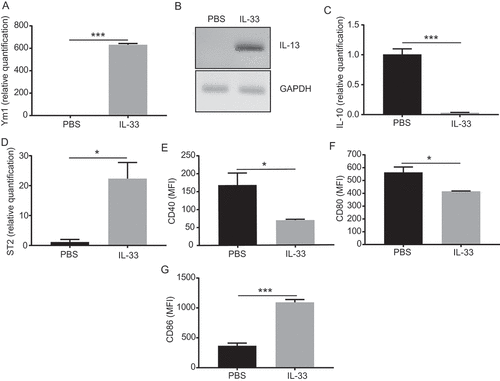
Figure 6. Maintenance of the Th2 response is necessary for the IL-33 mediated delay tumor progression in ovarian cancer.