Figures & data
Figure 1. Evaluation of CD40 receptor expression in clinical specimens.
(a) Representative examples of CD40-specific positive and negative immunohistochemical staining. (b) Cumulative results of CD40 expression in tumor specimens included in the TMA. Samples were considered “positive” if ≥10% of the total cell number showed evidence of moderate/strong CD40-specific staining. (c) CD40 expression on tumor and non-malignant stromal cells was differentially analyzed in clinical samples derived from breast, ovarian, and lung cancers. Data refer to percentages of tumor or stromal cells showing evidence of moderate/strong CD40-specific staining.
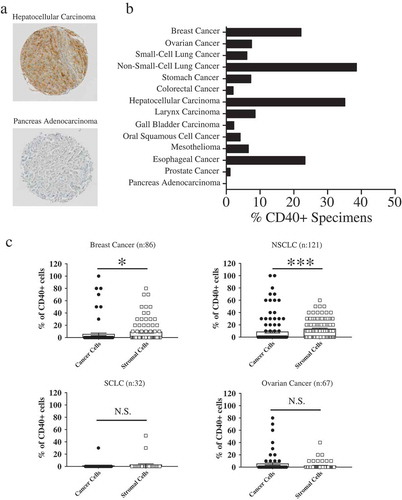
Figure 2. CD40L-expressing recombinant vaccinia virus treatment induces cytotoxic effects on CD40+ tumor cells in ‘“vitro’”.
Established tumor cell lines from (a) melanoma (Na8 and A375), (b) breast cancer (MDA-231 and BT-474), (c) colorectal cancer (HCT116 and LS180), and (d) hepatocellular carcinoma (PLC, HepG2 and HuH-7) were left untreated or infected with CD40L-expressing recombinant vaccinia virus (rVV40L) or vaccinia virus wild-type (VV WT) at MOI of 10. Tumor cells were also treated with soluble CD40L recombinant protein (s40L) and oligomerizing enhancer (0.5 and 1 μg/ml, respectively) alone or following VV WT infection (VV WT). On d 4, cells were collected and stained with annexin V and propidium iodide (PI) (a, b, c, and d). Data refer to a representative experiment for each established tumor cell line (upper panels) and report collective results (mean ± SEM) from five different experiments (lower panels). *P < 0.05, **P < 0.01; Mann–Whitney nonparametric test.
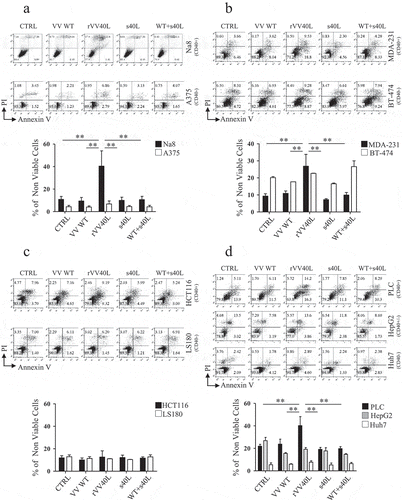
Figure 3. Lack of sensitivity to tumor cell death following rVV40L infection is associated with impaired CD40 signaling pathway.
Established melanoma (Na8 and A375) (a), breast cancer (MDA-231 and BT-474) (b), colorectal cancer (HCT116 and LS180) (c), and hepatocellular carcinoma (PLC, HepG2 and HuH-7) (d) cell lines were left untreated or infected with CD40L-expressing recombinant vaccinia virus (rVV40L) or vaccinia virus wild-type (VV WT) at an MOI of 10. In addition, cells were also treated with soluble CD40L recombinant protein (s40L) and oligomerizing enhancer (0.5 and 1 μg/ml, respectively) alone or following VV WT infection (VV WT), as indicated. After 4 d, TRAF-1 gene expression was evaluated by RT-qPCR. HCT116 (CD40+) colorectal cancer and PLC (CD40+) hepatocellular carcinoma cell lines were similarly treated, and NORE1A gene expression was assessed by RT-qPCR (e). Data are expressed as fold increase as compared to untreated tumor cells (n = 5 A, B, C, D and n = 3 E). *P < 0.05, **P < 0.01; Mann–Whitney nonparametric test.
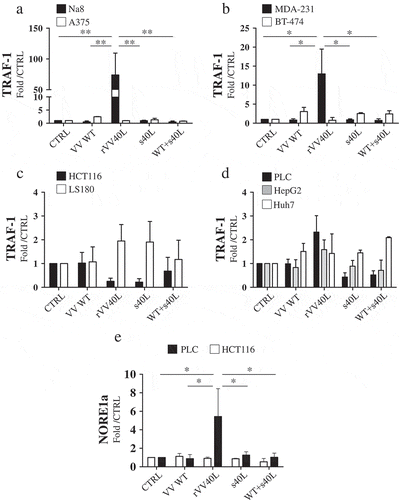
Figure 4. rVV40L infection modulates functional profiles of M1-/M2-like CD14-derived macrophages.
(a) Expression of CD40 on surfaces of CD14+ cell-derived M1- or M2-like macrophages was evaluated by flow cytometry. The left panel shows data from one representative experiment, whereas cumulative data from eight experiments with cells from different healthy donors are reported on the right panel. (b) Peripheral blood CD14+ monocytes from healthy donors were infected with rVV40L or with VV WT at MOI of 5 or treated with s40L and enhancer alone or following VV WT infection (WT + s40L). Cells were then cultured in the presence of GM-CSF or M-CSF. Culture supernatants were collected at the indicated time points, and cytokine release was assessed by ELISA. Data refer to cumulative results from eight (a) or four (b) independent experiments. **P < 0.01: Mann–Whitney nonparametric test.
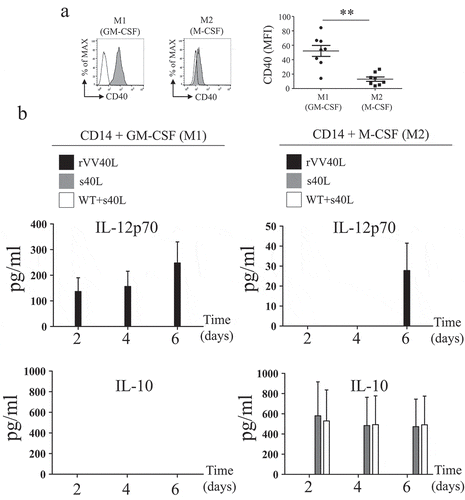
Figure 5. Co-culture with rVV40L-infected tumor cells promotes cytokine release and antitumor effects in polarized macrophages.
(a) M1- and M2-like macrophages were co-cultured (1:1 ratio) with LS180 CRC cells infected with VV WT or rVV40L (MOI 10). After 4 d, supernatants were collected and TNF-α, IL-12p70, IL-6, and IL-10 release was evaluated by ELISA. (b) M1- and M2-like macrophages were co-cultured with LS180 CRC cells, treated as described above, at the indicated ratios. On d 4, proliferation of LS180 CRC cells was evaluated by 3H-thymidine incorporation and expressed as percentage of proliferation as compared to untreated tumor cells. (c) Proliferative capacity of LS180 CRC cells, treated as described in (a), upon co-culture with M1- and M2-like macrophages, was evaluated by 3H-thymidine incorporation in the presence of neutralizing of anti-TNF-α mAb at 10 μg/ml concentration. Data refer to cumulative results from four independent experiments. *P < 0.05: Mann–Whitney nonparametric test.
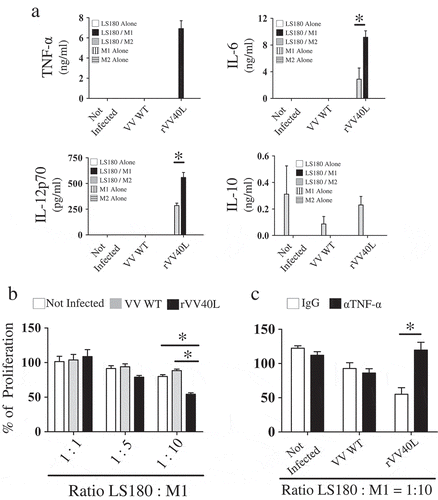
Figure 6. rVV40L induces CXCL10-mediated recruitment of CD8 + T cells and promotes cross-presentation of tumor-associated antigens by M1-like macrophages.
(a) M1- or M2-like macrophages were co-cultured at 1:1 ratio with HuH-7 hepatocellular (upper panel) or LS180 colorectal cancer (lower panel) cells untreated or infected with VV-WT or rVV40L at MOI 10. After 4 d, supernatants were collected and CXCL10 release was evaluated by ELISA. (b) CD8+ T-cell migration, induced by supernatants from co-cultures of M1-/M2-like macrophages with differentially infected tumor cells, was analyzed in the presence or absence of a neutralizing anti-CXCL10 mAb, as evaluated by flow cytometry. (c) Cross-presentation of MelanA/Mart-127–35 epitope from HLA-A0201(-) CD40(-) HT29 CRC cell lines co-infected with VV WT or rVV40L and with an rVV encoding MelanA/MART-1 full gene (rVVMART1 FG); 24h after infection, HT29 were co-cultured at 1:1 ratio with HLA-A0201+ M1- or M2-like macrophages. After 48 additional hours, 30,000 lymphocytes from a MelanA/Mart-127–35-specific, HLA-A0201-restricted CD8+ T-cell clones (see supplementary Figure 4) were added to the cultures, and IFN-γ release was evaluated by ELISA 48 hours later (*P < 0.05; Mann–Whitney nonparametric test).
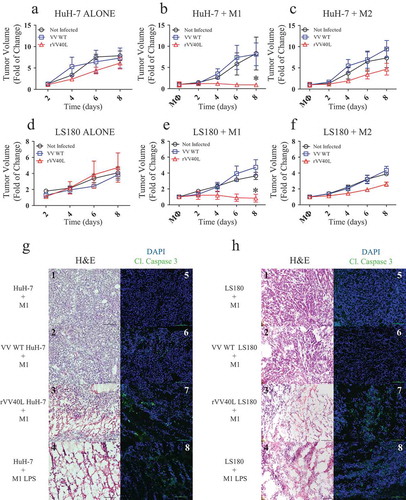
Figure 7. rVV40L promotes the antitumor activity of M1 macrophages in vivo.
105 LS180 CRC or HuH-7 HCC cells were inoculated subcutaneously (s.c.) into the flanks of NSG mice. Established tumors were then injected with PBS or 107 pfu of replication-incompetent VV WT or rVV40L; 48 hours later, 5 × 105 M1- or M2-like macrophages were injected into the tumor masses. Tumor volume of LS180 (a–c) and Huh7 (b–f) is expressed as fold increases as compared to tumor volumes measured at the time of macrophage inoculation. Data refer to two independent experiments with three mice in each condition. On d 8, mice were sacrificed and histology (H&E) and cleaved caspase 3 expression (green) were evaluated in LS180 (g) and HuH-7 (h) tumors. Data refer to representative stainings out of two tumors per condition displaying similar results (magnification 20x; scale bar 100 µm) (*P < 0.05; Mann–Whitney nonparametric test).