Figures & data
Figure 1. Duration between neoadjuvant immunotherapy and removal of primary tumor impacts on long-term survival.
(a-b), Groups of BALB/c WT mice (n = 5–10/grp) were injected with 5 × 104 4T1.2 mammary carcinoma cells into the mammary fat pad. a, b, As indicated, some groups of mice were treated i.p. with anti-PD-1+anti-CD137 or cIg on days 12 and 14. (a), Additionally, some groups received neoadjuvant anti-PD-1+anti-CD137 or cIg on days 6 and 8 (early). All neoadjuvant-treated groups had their primary tumors resected on day 16. Additionally, one group of mice was resected of their tumors on day 10 and treated i.p with adjuvant anti-PD-1+anti-CD137 on days 12 and 14. (b), Additionally, some groups of mice were treated with neoadjuvant anti-PD-1+anti-CD137 or cIg on days 6 and 8 (early) with primary tumors resected either on day 10 or 16. (c) Groups of BALB/c WT mice (n = 10/grp) were injected with 2 × 104 4T1.2 mammary carcinoma cells into the mammary fat pad. As indicated in the schematic, some groups of mice were treated i.p. with either anti-PD-1+anti-CD137 or anti-PD-1 alone or cIg on days 12 and 14 while other groups received neoadjuvant anti-PD-1+anti-CD137 or anti-PD-1 alone on days 14 and 16 (close). All groups had their primary tumors resected on day 16 as indicated in the schematic. (a,b,c) The Kaplan–Meier curves for overall survival of each group are shown. Significant differences between indicated groups were determined by log-rank sum test with exact p values shown. All experiments were performed once.
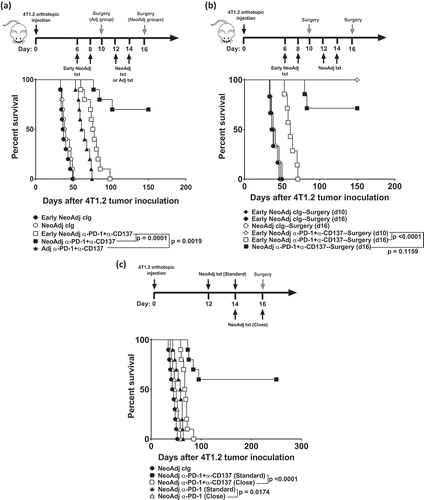
Figure 2. Duration between neoadjuvant immunotherapy and removal of primary tumor does not affect peripheral blood tumor-specific T cell expansion kinetics but attenuates its effector function.
(a), In an experimental setup similar to , peripheral blood was collected from all groups of mice (n = 5/grp) at the indicated time point and analyzed using flow cytometry. Gating on live CD45.2+ cells of lymphocyte morphology, the proportion of gp70 tetramer+ CD8+ TCRβ+ T cells are shown. Differences between neoadjuvant anti-PD-1+anti-CD137 (day 16) and early neoadjuvant anti-PD-1+anti-CD137 (day 10) (i.e. 4 days after the start of their respective therapies) were determined by unpaired Welch’s t-test with exact p-value indicated. (b), As indicated, groups of mice were treated i.p. with neoadjuvant anti-PD-1+anti-CD137 on days 11 and 13. Some groups of mice had their primary tumors resected on day 16 (surgery) compared to others that did not (No Surgery). Peripheral blood was collected from all groups of mice at the indicated time point, and the proportion of gp70 tetramer+CD8+TCRβ+ cells are shown. Differences between neoadjuvant anti-PD-1+anti-CD137 with and without surgery at day 15 were determined by unpaired Welch’s t-test with exact p-value indicated. (a-b), Data presented as mean + SEM with one naive mouse included in both experiments. Experiments were performed once. (c), In a similar experimental setup as , sorted lung gp70 tetramer+ CD8+ TCRβ+ T cells (n = 8–10 mice/group with 2 lungs pooled for each sample displayed) from all groups of mice on day 20 after tumor inoculation were re-stimulated with PMA/Io for 4 h and assessed for intracellular IFNγ+ production. Data presented as mean ± SEM. Differences between neoadjuvant anti-PD-1+anti-CD137 and early neoadjuvant anti-PD-1+anti-CD137 were determined by unpaired Welch’s t-test with exact p-value indicated. Data pooled from 2 experiments.
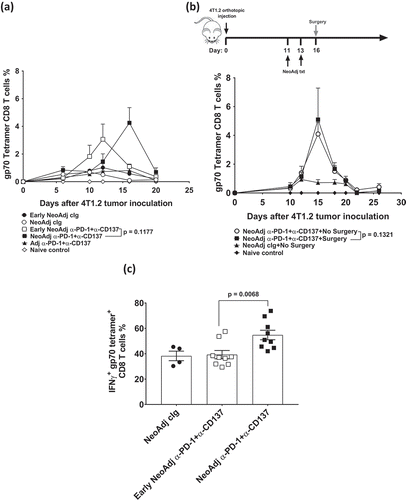
Figure 3. The blockade of lymphocyte migration attenuates the anti-tumor efficacy of neoadjuvant immunotherapy.
Groups of BALB/c WT mice (n = 5/grp) were injected with 5 × 10Citation4 4T1.2 mammary carcinoma cells in the mammary fat pad on day 0. a, As indicated in the schematic, all mice were treated i.p. with neoadjuvant anti-PD-1+anti-CD137 mAb on days 11 and 13 with all primary tumors resected on day 16. Additionally, these groups of mice were treated i.p. with either PBS or FTY720 every day from day 10 to day 16. (a) The Kaplan–Meier curves for overall survival of each group are shown. Data pooled from 2 experiments with significant differences between indicated groups determined by log-rank sum test with exact p values shown. (b-f) In a similar experimental setup as , all mice (n = 4–5/grp) were sacrificed on day 16 and their organs were collected and single cell suspensions generated for flow cytometry. Gating on live CD45.2+ cells of lymphocyte morphology, the absolute numbers of gp70 tetramer+ CD8+ TCRβ+ cells in the tumor (b), the draining lymph node (dLN) (c), the lung (d), the spleen (e) and the blood (f) are shown. Each symbol represents a single mouse. Data presented as mean ± SEM. Data pooled from 2 experiments with significant differences determined by unpaired Welch’s t-test with exact p-value shown.
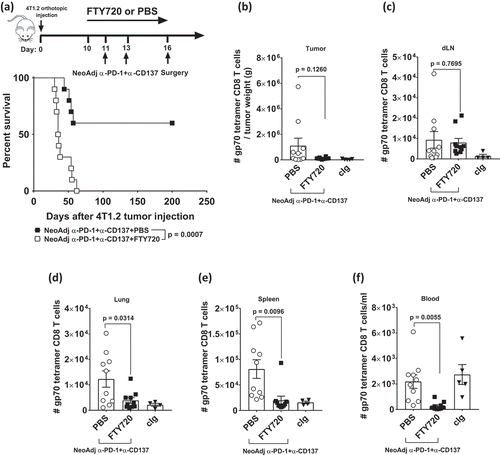
Figure 4. Comprehensive compared to standard neoadjuvant anti-PD-1+anti-CD137 are equally efficacious in impacting on long-term overall survival.
(a-b) Groups of BALB/c WT mice were injected with 5 × 10Citation4 4T1.2 mammary carcinoma cells into the mammary fat pad (n = 10/grp). As indicated, groups of mice were treated i.p. with neoadjuvant or adjuvant anti-PD-1+anti-CD137 on days 12 and 14 with all primary tumors resected on day 16 (neoadjuvant groups) or day 10 (adjuvant groups). Additionally, some groups of neoadjuvant and adjuvant-treated mice received additional anti-PD-1+anti-CD137 or cIg on days 18, 20, 27 and 34 (comprehensive). (b) In an experimental setup similar to (a), peripheral blood was collected from all groups of mice (n = 5/grp) at the indicated time point for flow cytometry. Gating on live CD45.2+ cells of lymphocyte morphology, the proportion of gp70 tetramer+ CD8+ TCRβ+ cells is shown. Data presented as mean + SEM. A naive mouse was also included in this experiment. Differences between mice that received standard or comprehensive anti-PD-1+anti-CD137 on day 20 following tumor inoculations were determined by unpaired Welch’s t-test with exact p-value indicated. c, Groups of C57BL/6J WT mice (n = 10/grp) were injected with 2 × 104 E0771 mammary carcinoma cells into the mammary fatpad. As indicated, some groups of mice were treated i.p. with neoadjuvant anti-PD-1+anti-CD137 mAb or cIg on days 8 and 10 (early), days 14 and 16 or days 14, 16, 20, 27 and 34 (comprehensive) with all primary tumors resected on day 18. One group of mice was treated with adjuvant anti-PD-1+anti-CD137 on days 20, 27 and 34 (comprehensive) with primary tumors resected on day 18. d, Groups of BALB/c WT mice (n = 5–10/grp) were injected with 2 × 10Citation4 4T1.2 mammary carcinoma cells into the mammary fat pad. As indicated, some groups of mice were treated i.p. with neoadjuvant anti-PD-1 alone, anti-PD-1+anti-CTLA4 or cIg on days 12 and 14 with all primary tumors resected on day 16. Additionally, some groups of neoadjuvant-treated mice received additional anti-PD1+anti-CTLA4 or cIg on days 18, 20, 27 and 34 (comprehensive). Other groups of mice received comprehensive adjuvant anti-PD-1 alone or anti-PD-1+anti-CTLA4 on days 18, 20, 27, 34 with primary tumors resected on day 16. (a, c, d) The Kaplan–Meier curves for overall survival of each group are shown. Significant differences between indicated groups were determined by log-rank sum test with exact p values shown. All experiments were performed once except for (d) where the data was pooled from 3 experiments. (b) is representative of two independent experiments.
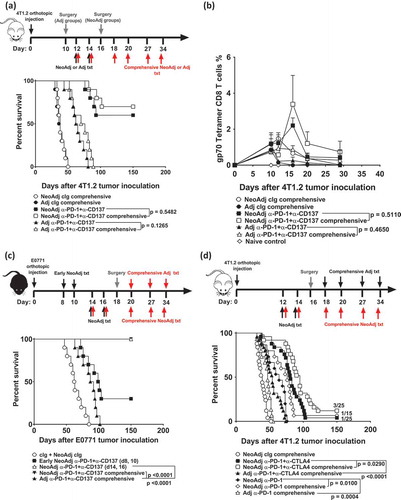
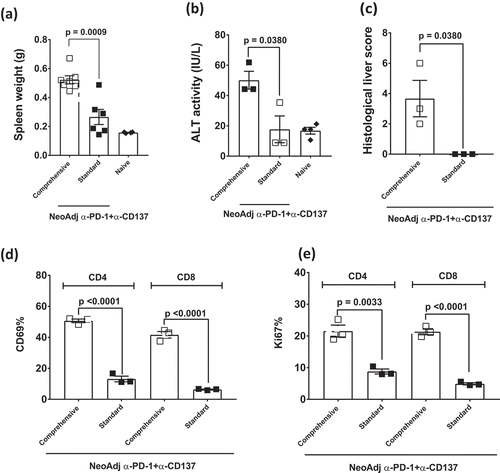
Figure 5. Comprehensive compared to standard neoadjuvant anti-PD-1+anti-CD137 immunotherapy increases the severity of immune-related adverse events.
(a-e), From the same experiment as and/or another experiment in a similar setup as , long-term surviving mice (n = 3–4/grp) treated with 2 doses (Standard) or 6 doses (Comprehensive) of neoadjuvant anti-PD-1+anti-CD137 were sacrificed on day 63 after 4T1.2 tumor inoculation. Naive mice (n = 2–4/grp) were included in the experiment. Spleens were collected and single cell suspensions generated for flow cytometry. Spleen weights (a), sera ALT levels (b) and histological liver score (c) of the indicated groups of mice are shown. Gating on live CD45.2+ cells of lymphocyte morphology, the proportions of splenic CD4+ and CD8+ T cells (TCRβ+) that were (d) CD69+ or (e) Ki67+ are shown. (a-e) Data presented as mean ± SEM. Significant differences between standard and comprehensive neoadjuvant-treated groups determined by unpaired Student’s t-test with exact p-value shown. All experiments were performed once except for (a) which was pooled from two experiments.