Figures & data
Figure 1. Expansion of γδ T cells from NB patient-derived PBMCs. (a) γδ T cells were expanded using serum-free conditions from commercially available healthy donor PBMCs (n = 2, with one repeated expansion using the same donor) or NB patients (n = 6, where some patient samples were expanded multiple times). All cultures were supplemented with IL-2 on days 0 (500 IU/mL), 3 (500 IU/mL), 6 (1,000 IU/mL), and 9 (1,000 IU/mL) and zoledronic acid (5 µM) on days 0 and 3. Live cells were gated for CD3+, CD56-, and pan γδ TCR+ to determine γδ T-cell percentage. Data comparing a healthy donor versus a NB patient starting product were not significantly different by non-paired, two tail t-test on day 7 (p = 0.97) and 12 (p = 0.55). (b) An apheresis product from a single NB patient was divided into two samples and each was expanded three times. The average γδ T-cell percentage and standard deviation are shown. (c) The cellular distribution of a starting PBMC product on day 0 differs from the cellular components on day 14 of expansion, and the change is not dependent on the donor being healthy or having NB. γδ T cells comprise less than 5% of the starting cellular product and increases to 60–90% of the expanded cells.
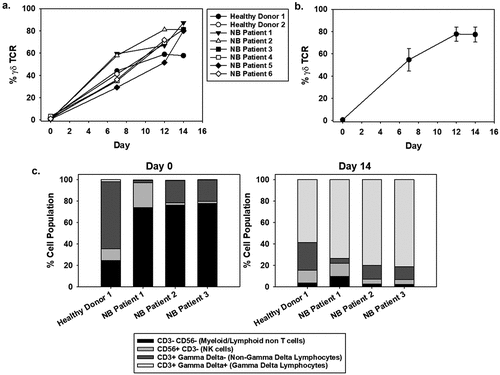
Figure 2. Cytotoxic activity of NB patient-derived γδ T cells Healthy donor (a, b) and NB patient (c, d) γδ T cells were expanded to day 14 and frozen at 1 × 107 cells/mL. Prior to freezing, cells were tested for cytotoxic potential against K562 cells. The left side panels show data from cells prior to freezing and the right side panels show data from cells after freezing. For the post-thaw samples, cells were thawed at 37oC and incubated in growth media for 8 hrs prior to use in the 4 hr cytotoxicity assay. (b, d) γδ T cells were used at a 5:1 effector to target ratio and incubated for 4 hr with K562 cells. Cytotoxicity was measured using flow cytometry by gating on the target cells stained with VPD450 and then analyzing the target cells for the dead stain dye, eFluor Alexa 780. There was no significant difference between pre-freeze and post-thaw γδ T-cell percentage as well as cytotoxicity (one-way ANOVA p = 0.618, N = 4). Raw representative flow cytometry data are shown.
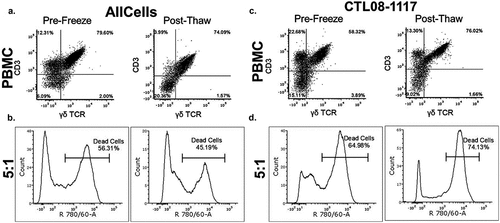
Figure 3. CD16 expression of NB patient-derived γδ T cells and dinutuximab binding to NB cell lines A. γδ T cells were expanded using serum-free conditions and examined for surface expression of CD16 by flow cytometry. CD16 expression on days 0, 6, 12, and 14 of expansion is shown for the bulk culture (a) and specifically on γδ T cells (b). Multiple NB cell lines of variable genomic profiles and disease aggressiveness were evaluated for the percentage of GD2 surface expression and dinutuximab (DTX) binding (c) Biotinylated DTX (0.5 μg) was used to demonstrate the specificity of antibody binding to NB cell lines, which showed a trend between GD2 expression and DTX binding with increased surface GD2 corresponding to increased DTX binding (N = 3 per sample and the mean is shown).
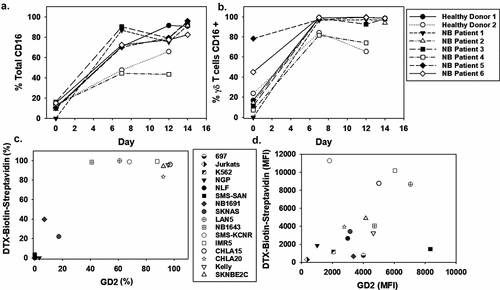
Figure 4. Evaluating ADCC with NB patient-derived γδ T cells (a) Cytotoxicity assay using NB cell lines that showed high GD2 expression. Black bars indicate NB patient-derived γδ T cells at a 5 to 1 effector to target ratio while the grey bars represent the same assay with dinutuximab (DTX) included at 5 μg/mL. DTX alone did not show significant killing (data not shown). Expanded NB patient cells functioned as the effector cells and experiments were repeated in triplicate, mean and standard deviations (SD) are shown. (Significance determined by paired t-test * p < 0.05; **p < 0.01). (b) Live cell images at 6 hrs of IMR5 cells (stained with CFSE) co-incubated with or without γδ T cells (stained with VPD450 proliferation dye) and with or without DTX (5 μg/mL). Cell death was quantified by PI intensity (100 μM). The mean and SD (N = 4) were quantified by the relative intensity of PI staining over 6 hrs during live cell imaging. (c) Representative still images at 6 hrs: CFSE stain shows the IMR5 target cells, VPD450 shows the effector γδ T cells, PI staining identifies dead cells and bright field are captured.
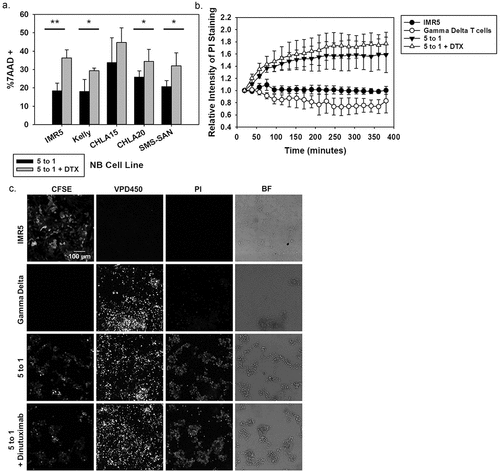
Figure 5. In vivo effectiveness of NB patient-derived γδ T cells and dinutuximab (a) Schematic of the treatment plan for NSG mice injected subcutaneously with IMR5 cells and treated with γδ T cells, dinutuximab (DTX), or a combination of the two. (b) The mean tumor volume is shown for each group: untreated (N = 8), γδ T cells only (N = 8), γδ T cells + DTX (200 μg) (N = 6), γδ T cells + DTX (400 μg) (N = 4). The mean and standard deviation is shown for each cohort. (c) NSG mice with established IMR5 subcutaneous tumors were injected with 200 μg biotinylated DTX. Mice were sacrificed and tumors processed prior to injection (untreated) and after injection on days 1, 4, or 7. Representative flow cytometry for N = 3 is shown.
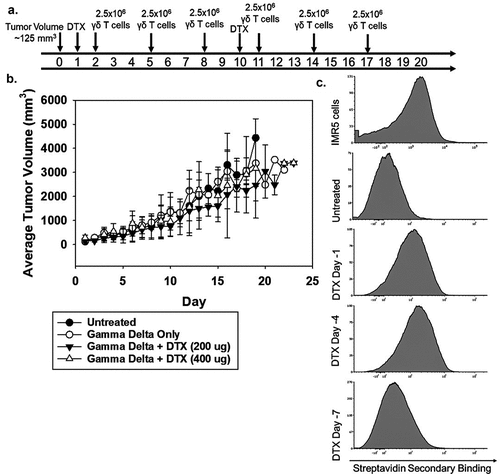
Figure 6. Increasing γδ T-cell function when combined with dinutuximab and TMZ (a) Representative flow cytometry of IMR5 cell killing with combinations of dinutuximab (DTX), TMZ, and γδ T cells. From top to bottom, panel A shows killing for non-treated cells or cells treated with DTX, TMZ, γδ T cells (5:1 effector:target), γδ T cells (5:1 effector:target) with DTX, or γδ T cells (5:1 effector:target) with DTX and TMZ. IMR5 background dead cell staining was 6%, which was similar to treatments with DTX (5 μg/mL; 11% dead cells) and TMZ (400 μM; 11% dead cells). Average cell death increased to approximately 30% with γδ T cells, 39% with γδ T cells and DTX, and 46% with γδ T cells, DTX, and TMZ (N = 3, p < 0.05). (b) Average and SD (N = 3) of human cytokine array quantified using ImageJ analysis. Consistent with previous findings, γδ T cells secrete proinflammatory cytokines IFNγ and TNFα mixed with NB cells. There is an increase in cytokine production when DTX and TMZ is included. All data is quantified as relative intensity to naïve γδ T cells. There is no significant difference between effector to target ratios 5 to 1 + DTX and 5 to 1 + DTX + TMZ. Data in these groups is significantly different compared to 5 to 1 effector to target ratio alone p < 0.05. (c) Flow cytometry was performed to measure FASL and CD107a on γδ T cells 4 hrs after initiation in cytotoxicity assays. Black and grey bars represent FASL and CD107a, respectively. Expression of these proteins is elevated when γδ T cells are incubated with IMR5 cells and further elevated when combined with DTX and TMZ.
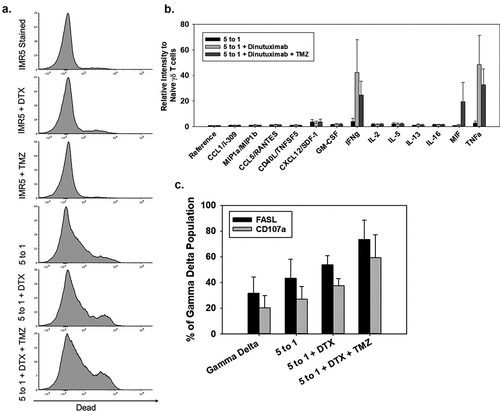
Figure 7. Enhancing NB patient-derived γδ T-cell effectiveness in vivo by combination therapy (a-b) Schematic representation of the treatment plan for NSG mice injected subcutaneously with IMR5 cells. Time T = 0 refers to when the tumor reaches a minimum of 125 mm3 and the start of treatment. NSG mice with established IMR5 subcutaneous tumors were treated over a 17-day period at varied doses of TMZ, DTX, and γδ T cells. (c) Tumor volume was measured and average tumor volume over time and standard deviation was calculated for TMZ doses of 20 mg/kg (N = 4), 40 mg/kg (N = 5), 60 mg/kg (N = 2), 85 mg/kg (N = 5), and compared to untreated controls. (d) Untreated (N = 8), 2.5 × 106 γδ Only (N = 8), γδ + dinutuximab (DTX) (400 μg) [N = 4], TMZ (40 mg/kg) [N = 5], DTX (400 μg)+TMZ (40 mg/kg) [N = 4], γδ+TMZ (40 mg/kg) [N = 5], and γδ+DTX (400 μg)+TMZ (40 mg/kg) [N = 6] were evaluated through day 30. (e) A lower dose of TMZ (20 mg/kg) [N = 4] was used alone or with various combinations of DTX and γδ T cells [minimum of N = 4 per cohort]. (f) TMZ (40 mg/kg) [N = 5] and γδ+DTX (400 μg) + TMZ (40 mg/kg) [N = 6] are compared by paired t-test over 4 weeks (*p = 0.029 at week 4). (g) Survival curves to day 50 from the start of treatment, shows a significant survival advantage among animals that received 40 mg/kg TMZ with 400 μg DTX and 2.5 × 106 γδ T cells compared to γδ T cells alone, γδ T cells + DTX, γδ T cells + TMZ, and TMZ + DTX (log-rank p < 0.001). (h) Survival curves to day 50 from the start of treatment, demonstrates significance in survival when using lower doses of TMZ (20 mg/kg) with 400 μg DTX and 2.5 × 106 γδ T cells compared to untreated animals, γδ T cells only, γδ T cells+DTX, and TMZ (20 mg/kg) only (log-rank p < 0.001).
![Figure 7. Enhancing NB patient-derived γδ T-cell effectiveness in vivo by combination therapy (a-b) Schematic representation of the treatment plan for NSG mice injected subcutaneously with IMR5 cells. Time T = 0 refers to when the tumor reaches a minimum of 125 mm3 and the start of treatment. NSG mice with established IMR5 subcutaneous tumors were treated over a 17-day period at varied doses of TMZ, DTX, and γδ T cells. (c) Tumor volume was measured and average tumor volume over time and standard deviation was calculated for TMZ doses of 20 mg/kg (N = 4), 40 mg/kg (N = 5), 60 mg/kg (N = 2), 85 mg/kg (N = 5), and compared to untreated controls. (d) Untreated (N = 8), 2.5 × 106 γδ Only (N = 8), γδ + dinutuximab (DTX) (400 μg) [N = 4], TMZ (40 mg/kg) [N = 5], DTX (400 μg)+TMZ (40 mg/kg) [N = 4], γδ+TMZ (40 mg/kg) [N = 5], and γδ+DTX (400 μg)+TMZ (40 mg/kg) [N = 6] were evaluated through day 30. (e) A lower dose of TMZ (20 mg/kg) [N = 4] was used alone or with various combinations of DTX and γδ T cells [minimum of N = 4 per cohort]. (f) TMZ (40 mg/kg) [N = 5] and γδ+DTX (400 μg) + TMZ (40 mg/kg) [N = 6] are compared by paired t-test over 4 weeks (*p = 0.029 at week 4). (g) Survival curves to day 50 from the start of treatment, shows a significant survival advantage among animals that received 40 mg/kg TMZ with 400 μg DTX and 2.5 × 106 γδ T cells compared to γδ T cells alone, γδ T cells + DTX, γδ T cells + TMZ, and TMZ + DTX (log-rank p < 0.001). (h) Survival curves to day 50 from the start of treatment, demonstrates significance in survival when using lower doses of TMZ (20 mg/kg) with 400 μg DTX and 2.5 × 106 γδ T cells compared to untreated animals, γδ T cells only, γδ T cells+DTX, and TMZ (20 mg/kg) only (log-rank p < 0.001).](/cms/asset/a67315bc-0809-4e06-a9cc-5b4d86a22dfe/koni_a_1593804_f0007_b.gif)
