Figures & data
Figure 1. Optimizing CD8+ T cells for tumor cell cytotoxicity assays.
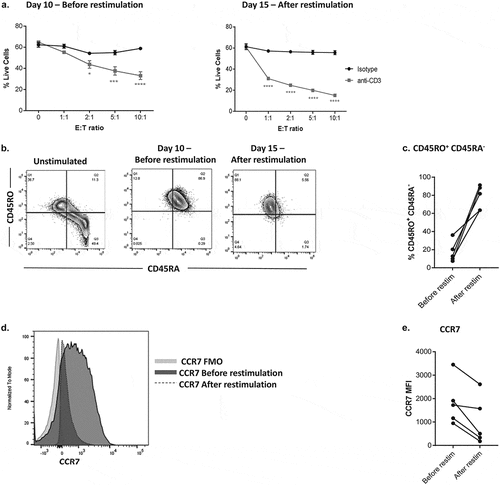
Figure 2. Generation of anti-CD3 expressing tumor cell lines.
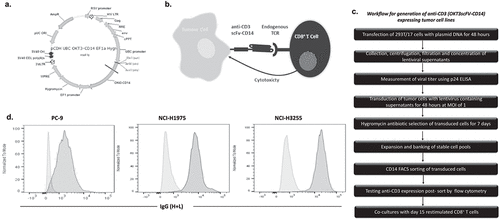
Figure 3. Co-culture of CD8+ T cells with tumor cells expressing anti-CD3 results in target cell T cell-mediated cytotoxicity.
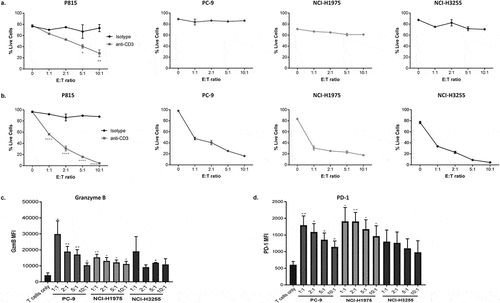
Figure 4. Expression levels of anti-CD3 influence the extent of tumor cell cytotoxicity by T cells.
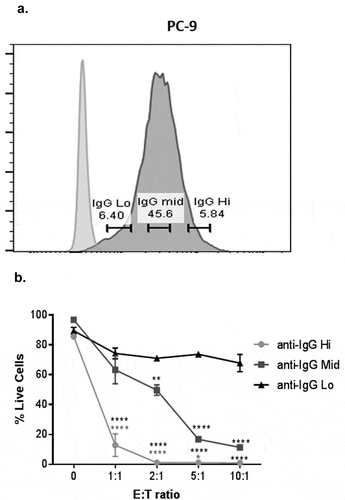
Figure 5. Measurements of IFN-ɣ and early tumor cell apoptotic events provide additional sensitive pharmacodynamic endpoints in this assay system.
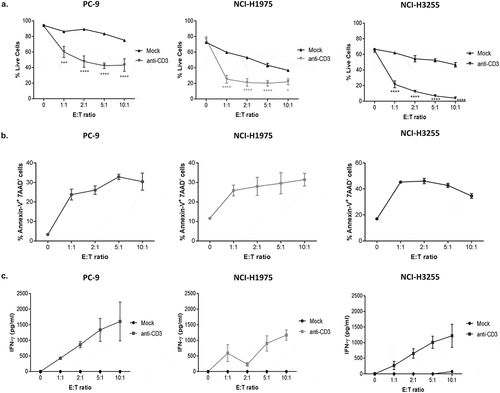
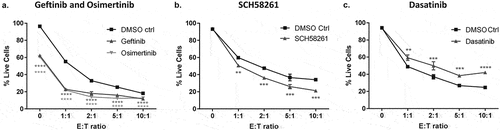
Figure 6. Investigating the impact of targeted therapies on T cell-mediated cytotoxicity using the anti-CD3-expressing tumor cell co-culture system.