Figures & data
Figure 1. Lentiviral transduction of primary canine T cells
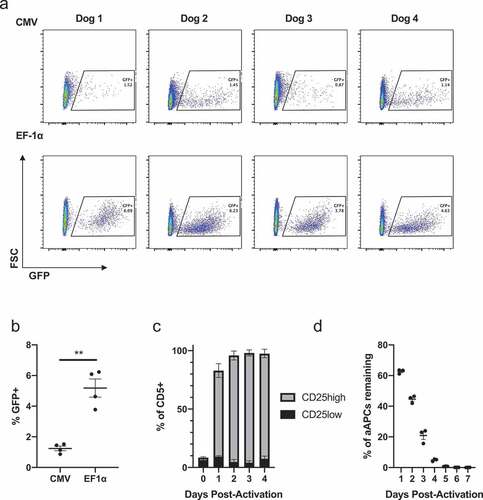
Figure 2. Design, manufacture and function of cCD20-28-ζcanine CAR T cells
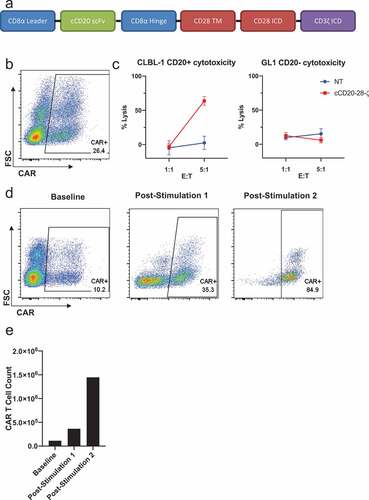
Table 1. cCD20-28-ζ: clinical parameters and CAR T cell characteristics
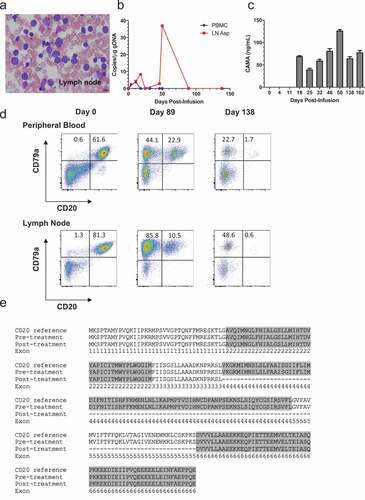
Figure 3. Treatment of patient 429–003 with cCD20-28-ζCAR T cells
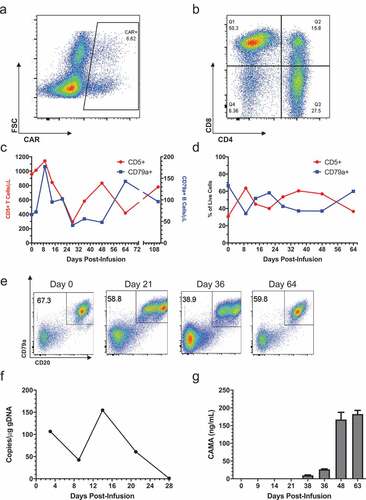
Figure 4. Design and expansion of cCD20-BB-ζ canine CAR T cells
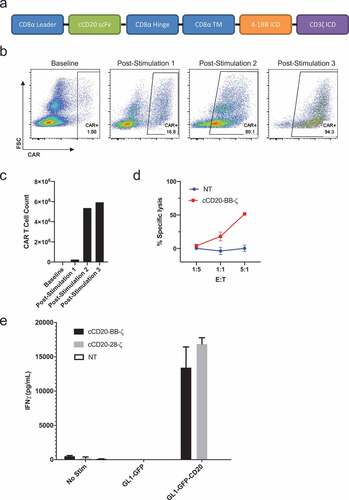
Figure 5. Treatment of patient 429–006 with cCD20-BB-ζ CAR T cells