Figures & data
Table 1. Characteristics of study participants
Figure 1. Study design. BC, breast cancer; NHC, normal human controls; BBD, benign breast disease; ELISA, enzyme-linked immunosorbent assay
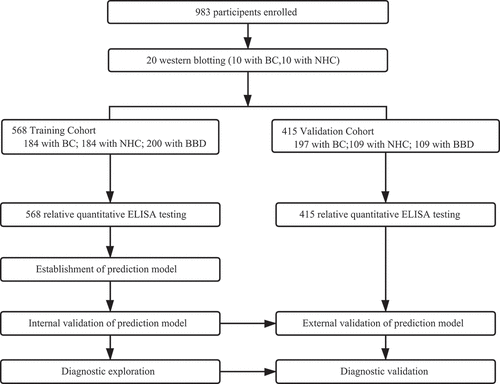
Figure 2. Profiles of autoantibodies against 11 tumor-associated antigens tested by western blotting in 10 patients with breast cancer (B1-B10) and 10 normal controls (C1-C10)
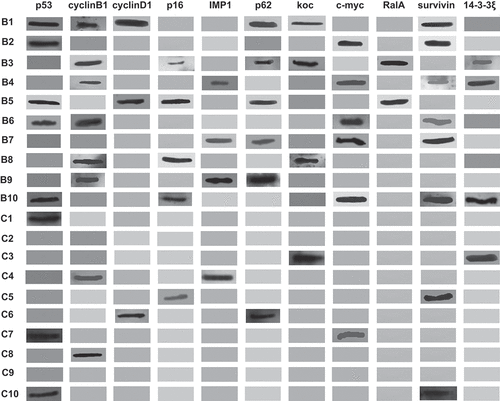
Table 2. Serum relative concentration levels of individual autoantibody among breast cancer patients, breast benign disease and normal individual
Figure 3. Scatter plots of sera relative concentration levels of individual autoantibody among breast cancer patients (NTC = 184, NVC = 197), benign breast disease (NTC = 200, NVC = 109) and normal individual (NTC = 184, NVC = 109) in two cohorts (TC, training cohort; VC, validation cohort). BC, breast cancer; NHC, normal human controls; BBD, benign breast disease
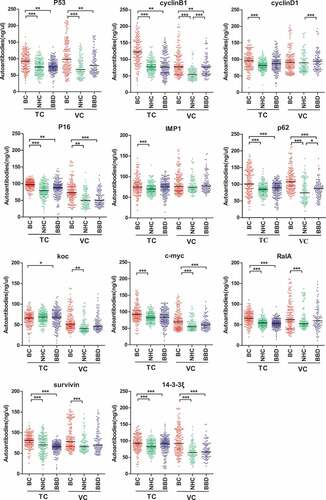
Table 3. LOOCV results predicted by the two models of BC
Figure 4. Performance of the prediction model with five anti-TAAs autoantibodies to detect breast cancer. Receiver operating characteristic (ROC) curves from training cohort (a, b) and validation cohort (c, d). Performance shown for distinguishing individuals with BC from NHC (a, c) or BBD controls (b, d). TC, training cohort; VC, validation cohort; BC, breast cancer; NHC, normal human controls; BBD, benign breast disease; Se, sensitivity; Sp, specificity
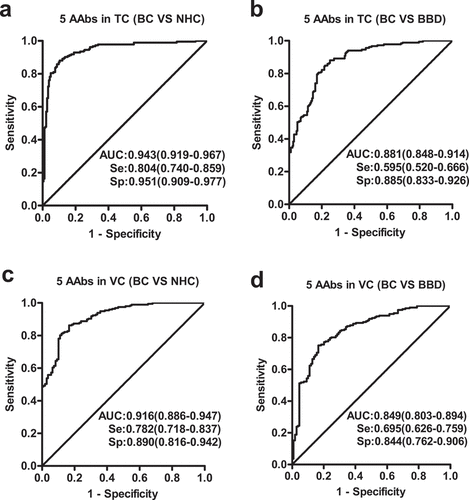
Table 4. Diagnostic value of the prediction model with five anti-TAAs autoantibodies for different stages and ages of BC patients
Figure 5. Positive rates of autoantibodies against eight TAAs or combined five TAAs in individual sera of BC patients, BBD patients and NHC (a, b). The cutoff value was set at the maximum Yoden index when the specificity was greater than 95%. Positive rates of the prediction model with five anti-TAAs autoantibodies among subgroups of BC patients based on tumor stage (c-f), molecular subtypes (g), lymph node metastasis (h), histological type (i) and family history of tumor (j). TC, training cohort; VC, validation cohort; BC, breast cancer; NHC, normal human controls; BBD, benign breast disease
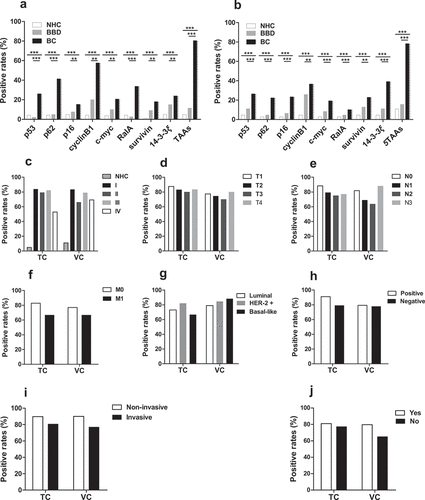
Table 5. Positive reactivity of the prediction model with five anti-TAAs autoantibodies among subgroups
